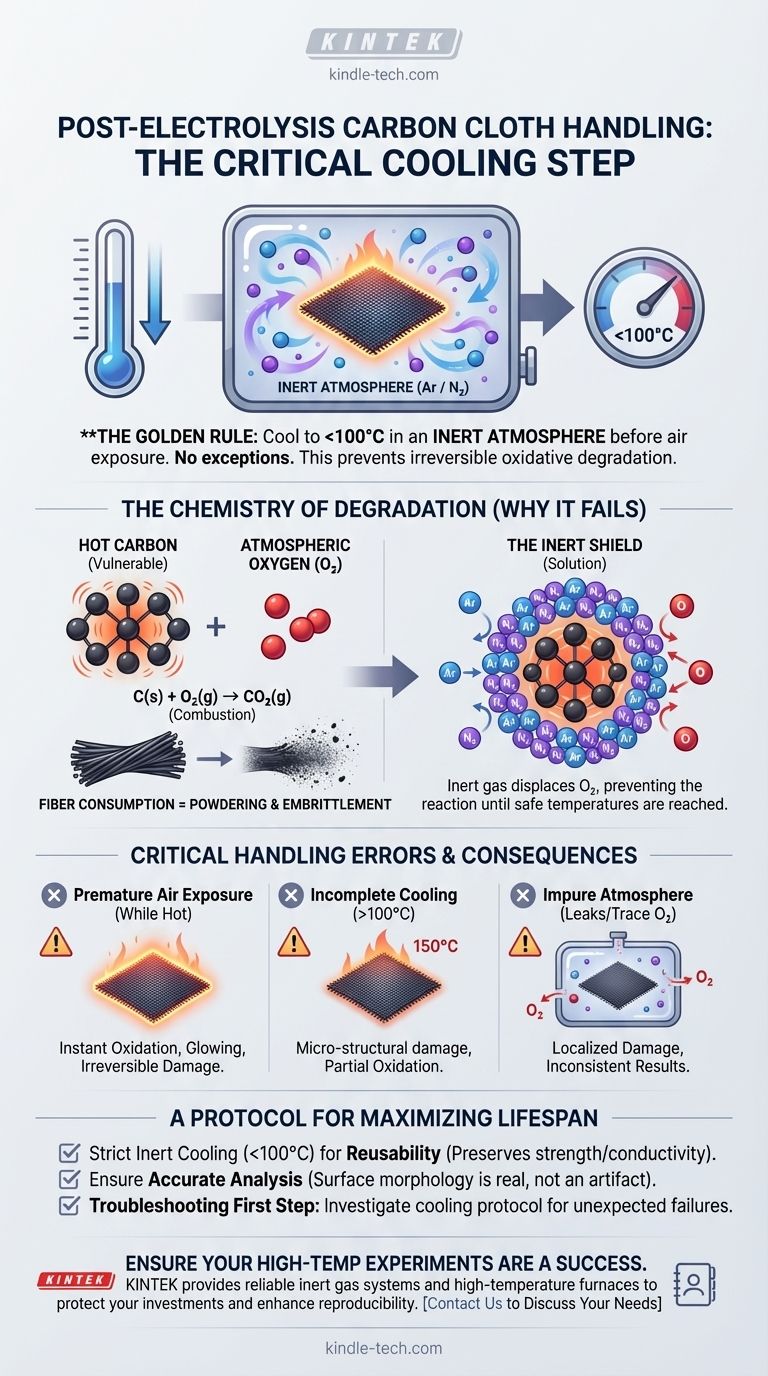
To preserve the integrity of carbon cloth after high-temperature electrolysis, it must be completely cooled to below 100°C within an inert atmosphere before any exposure to ambient air. This single, critical step is the only way to prevent immediate and irreversible oxidative degradation of the material.
The core principle is oxygen prevention. At elevated temperatures, the high-surface-area carbon fibers are extremely reactive with atmospheric oxygen. An inert atmosphere acts as a protective shield, preventing this chemical reaction during the material's most vulnerable state.

The Chemistry of Post-Electrolysis Degradation
Understanding the mechanism of failure is key to appreciating the necessity of a strict handling protocol. The problem is not mechanical but chemical, triggered by the combination of heat and oxygen.
Why Temperature is the Catalyst for Failure
Immediately following a high-temperature process, the carbon cloth possesses significant thermal energy. This energy dramatically lowers the activation barrier required for carbon to react with oxygen.
In this energized state, the material is exceptionally susceptible to chemical attack. It is not the same stable material you handled at room temperature.
The Role of Oxygen: From Fabric to Powder
When hot carbon is exposed to air, a rapid oxidation reaction occurs (C + O₂ → CO₂). This is a form of combustion.
This process is not a surface-level tarnish; it is a conversion of the solid carbon fibers into carbon dioxide gas. The structural backbone of the cloth is literally consumed, resulting in the physical "powdering" and embrittlement observed in mishandled samples.
The "Inert Atmosphere" Shield
An inert atmosphere, typically composed of gases like argon (Ar) or nitrogen (N₂), protects the carbon cloth by displacing oxygen.
By removing the key reactant (oxygen) from the environment, the oxidation reaction cannot proceed. This allows the cloth to cool down safely to a temperature where it is no longer chemically reactive with the air.
Critical Handling Errors and Their Consequences
Deviating from the correct procedure introduces variables that can compromise your results or destroy your material. Awareness of these common errors is crucial.
The Mistake of Premature Air Exposure
Removing the carbon cloth from the inert atmosphere while it is still hot is the most common and damaging error.
The material will begin to oxidize instantly. Depending on the temperature, this can range from rapid embrittlement to visible glowing or smoldering as the fibers burn away. This damage is irreversible.
The Flaw of Incomplete Cooling
The 100°C threshold is a conservative, safe target. While reactivity decreases as the material cools, it can still be significant at temperatures well above 100°C.
Failing to cool the material sufficiently before exposing it to air can still cause micro-structural damage and partial oxidation, compromising its performance in subsequent uses or analyses.
Assuming an "Impure" Inert Atmosphere is Sufficient
Leaks in your system or an incomplete purge of the chamber can allow trace amounts of oxygen to remain.
Even a small percentage of oxygen can cause significant localized damage to the hot carbon fibers. This can lead to inconsistent material properties and non-repeatable experimental results.
A Protocol for Maximizing Material Lifespan
Your post-process handling protocol should be as rigorous as your experimental procedure. Your goal determines which aspect of this process is most critical.
- If your primary focus is material reusability: Strict adherence to the inert atmosphere cooling protocol below 100°C is non-negotiable to preserve mechanical strength and electrical conductivity.
- If your primary focus is post-process analysis (e.g., microscopy): This procedure ensures the surface morphology you observe is a direct result of the electrolysis, not an artifact of post-process combustion.
- If you are troubleshooting unexpected material failure: Improper cooling should be the first variable investigated, as it is the most common cause of catastrophic degradation.
Controlling the environment after the experiment is what ensures the integrity and value of your results.
Summary Table:
| Critical Step | Purpose | Consequence of Error |
|---|---|---|
| Cool to <100°C in inert atmosphere | Prevents carbon oxidation (C + O₂ → CO₂) | Irreversible material degradation, powdering |
| Use pure Argon or Nitrogen | Displaces oxygen, the key reactant | Inconsistent results, localized damage |
| Ensure system is leak-free | Maintains a true inert environment | Partial oxidation, compromised performance |
Ensure your high-temperature experiments are a success. Proper handling of sensitive materials like carbon cloth is essential for accurate results and material longevity. KINTEK specializes in providing reliable lab equipment and consumables, including inert gas systems and high-temperature furnaces, to support your laboratory's precise needs. Contact us today to discuss how our solutions can protect your investments and enhance your research reproducibility.
Visual Guide
Related Products
- Conductive Carbon Cloth Carbon Paper Carbon Felt for Electrodes and Batteries
- Electrode Polishing Material for Electrochemical Experiments
- Conductive Boron Nitride BN Ceramics Composite for Advanced Applications
- Ultra-High Temperature Graphite Vacuum Graphitization Furnace
- Custom PTFE Teflon Parts Manufacturer for Hollow Etching Flower Basket ITO FTO Developing Glue Removal
People Also Ask
- Why are high surface area materials preferred for BES anodes? Maximize Microbial Power and Efficiency
- What are the four main types of sensors? A Guide to Power Source and Signal Type
- What applications is carbon felt suitable for? Ideal for High-Performance Electrochemical Systems
- What is the ideal operating environment for a glassy carbon sheet? Ensure Optimal Performance and Longevity
- What are 3 products that carbon nanotubes can be used in? Enhancing Batteries, Tires, and Composites



















