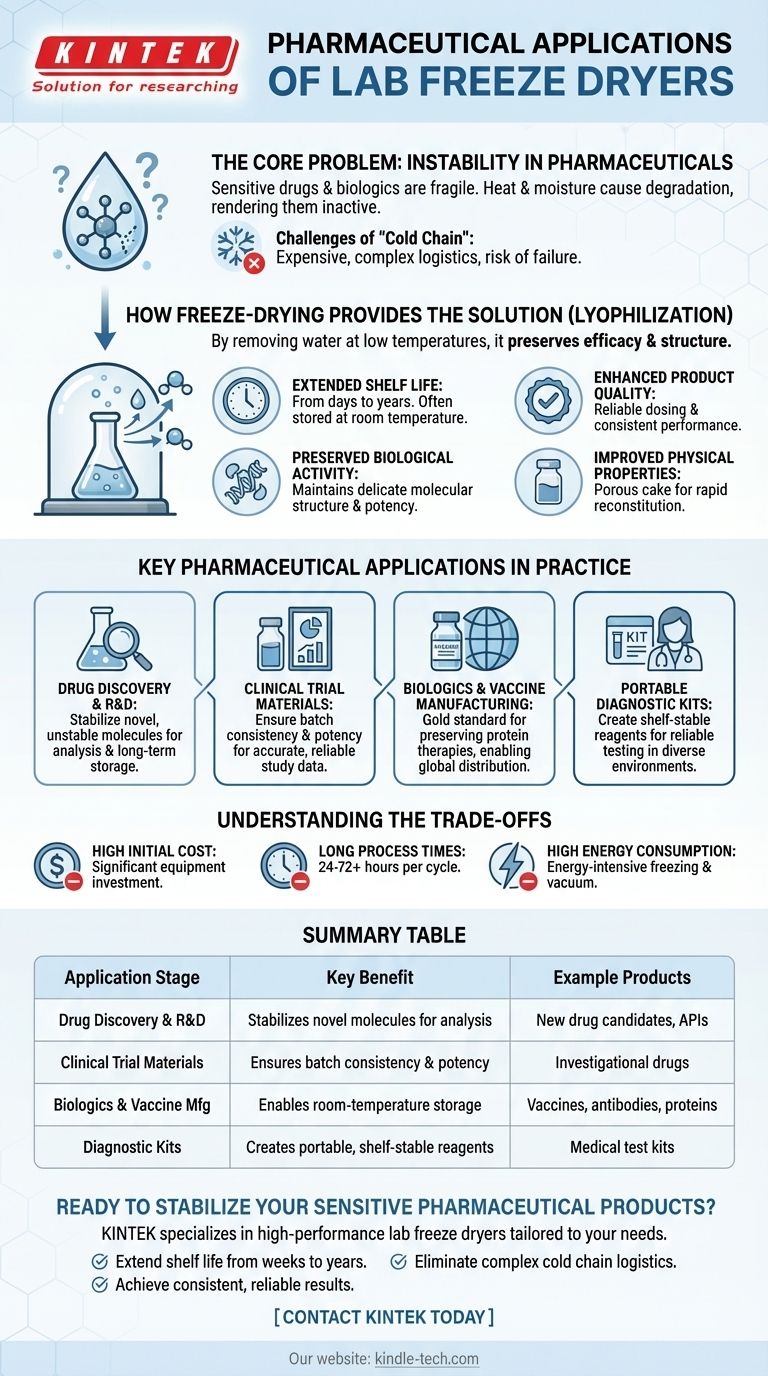
In the pharmaceutical industry, laboratory freeze dryers are essential tools for stabilizing sensitive drugs and biological materials. Their primary applications span the entire product lifecycle, from initial research and development of new drug candidates to producing stable investigational drugs for clinical trials and manufacturing small commercial batches of vaccines, proteins, and complex small-molecule drugs.
The core function of a lab freeze dryer in pharmaceuticals is to solve the fundamental problem of product instability. By removing water at low temperatures, it preserves a drug's efficacy and structure, dramatically extending its shelf life and simplifying storage and transportation.

The Core Problem: Instability in Pharmaceuticals
Many of the most advanced medicines, especially biologics, are inherently fragile. Their effectiveness depends on maintaining a precise molecular structure that can be easily compromised.
Why Liquid Formulations Degrade
In a liquid state, complex molecules like proteins and vaccines are susceptible to chemical and physical degradation. Heat and moisture can break down their structure, rendering them inactive and unusable in a short period.
The Challenge of the "Cold Chain"
To combat this degradation, many liquid biologics require a constant "cold chain"—an unbroken, temperature-controlled supply line from manufacturing to patient. This is logistically complex, expensive, and carries a high risk of failure if the temperature deviates.
How Freeze-Drying Provides the Solution
Lyophilization, or freeze-drying, directly addresses the root cause of instability by removing water, which is the primary medium for degradative chemical reactions.
Extending Shelf Life Dramatically
The most significant benefit is the extension of a product's shelf life from days or weeks to several years. Freeze-dried products can often be stored at room temperature, eliminating the need for a costly and rigid cold chain.
Preserving Biological Activity
The process is gentle enough to maintain the delicate three-dimensional structure of proteins, enzymes, and vaccine components. This ensures the product retains its full biological potency and therapeutic effect when it is reconstituted.
Enhancing Product Quality
By reducing moisture content to minimal levels, freeze-drying creates an exceptionally stable product. This stability translates to more reliable dosing, consistent performance, and a higher standard of quality control.
Improving Physical Properties
Freeze-drying creates a lightweight, porous cake that is easy to transport. This porous structure also allows for rapid reconstitution, meaning it can be quickly and completely dissolved back into a liquid for administration.
Key Pharmaceutical Applications in Practice
Lab freeze dryers are deployed at critical stages of the pharmaceutical pipeline, each leveraging the core benefits of lyophilization.
Drug Discovery and R&D
In early-stage research, scientists work with novel and often unstable molecules. Freeze-drying allows them to stabilize these new drug candidates for analysis, formulation studies, and long-term storage.
Clinical Trial Materials
For clinical trials, it is crucial that every dose of an investigational drug is consistent and potent. Freeze-drying ensures the stability of these materials, guaranteeing that trial results are accurate and reliable.
Biologics and Vaccine Manufacturing
Freeze-drying is the gold standard for preserving many vaccines, antibodies, and other protein-based therapies. It is essential for producing stable formulations that can be stockpiled and distributed globally, especially during health crises.
Portable Diagnostic Kits
Many medical diagnostic kits rely on sensitive biological reagents. Freeze-drying these components makes the kits shelf-stable and portable, allowing for reliable testing in diverse environments without the need for refrigeration.
Understanding the Trade-offs
While incredibly powerful, freeze-drying is not a universal solution and comes with practical considerations.
High Initial Cost
Lyophilization equipment represents a significant capital investment. The technology is complex, requiring precise control over temperature and vacuum systems.
Long Process Times
A typical freeze-drying cycle can take anywhere from 24 to 72 hours, or even longer. This makes it a time-consuming, batch-oriented process that can impact manufacturing throughput.
High Energy Consumption
The combination of deep freezing and pulling a high vacuum is energy-intensive. This contributes to higher operational costs compared to simpler liquid formulation processes.
Making the Right Choice for Your Goal
The decision to use a lab freeze dryer should align with the specific needs of your pharmaceutical product.
- If your primary focus is early-stage R&D: Use freeze-drying to preserve novel, sensitive molecules, ensuring you have stable material for foundational testing.
- If your primary focus is clinical trials: Employ freeze-drying to create uniform, stable batches that guarantee the integrity and reliability of your study data.
- If your primary focus is manufacturing a biologic: Leverage freeze-drying to ensure long-term product efficacy, simplify logistics, and eliminate the complexities of the cold chain.
- If your primary focus is developing diagnostics: Use freeze-drying to stabilize sensitive reagents, creating reliable and highly portable test kits for broad distribution.
Ultimately, freeze-drying empowers the pharmaceutical industry to transform fragile, complex molecules into stable, effective, and accessible medicines.
Summary Table:
| Application Stage | Key Benefit | Example Products |
|---|---|---|
| Drug Discovery & R&D | Stabilizes novel molecules for analysis | New drug candidates, APIs |
| Clinical Trial Materials | Ensures batch consistency and potency | Investigational drugs |
| Biologics & Vaccine Mfg | Enables room-temperature storage | Vaccines, antibodies, proteins |
| Diagnostic Kits | Creates portable, shelf-stable reagents | Medical test kits |
Ready to stabilize your sensitive pharmaceutical products?
At KINTEK, we specialize in providing high-performance lab freeze dryers tailored to the exacting demands of the pharmaceutical industry. Whether you are in drug discovery, developing clinical trial materials, or manufacturing biologics, our equipment ensures your products maintain their potency and stability.
We help you:
- Extend shelf life from weeks to years.
- Eliminate complex cold chain logistics.
- Achieve consistent, reliable results in every batch.
Let our experts guide you to the right lyophilization solution for your laboratory's needs. Contact KINTEK today to discuss how our lab equipment can enhance your pharmaceutical development and manufacturing processes!
Visual Guide
Related Products
- Benchtop Laboratory Freeze Dryer for Lab Use
- Laboratory Sterilizer Lab Autoclave Pulsating Vacuum Desktop Steam Sterilizer
- Low-Temperature Water-Cooled Touchscreen Vibratory Ultrafine Pulverizer
- Desktop Fast High Pressure Laboratory Autoclave Sterilizer 16L 24L for Lab Use
- Custom PTFE Wafer Holders for Lab and Semiconductor Processing
People Also Ask
- What are some other applications of freeze dryers? Preserving Delicate Materials in Tech and Research
- Why is manufacturer reputation important when selecting a lab freeze dryer? Ensure Long-Term Reliability for Your Samples
- What is the importance of ultimate vacuum in a freeze dryer? A Key Diagnostic for Efficient Drying
- What happens during the primary drying phase of freeze drying? Master the Sublimation Process
- What are the key factors that influence the price of a lab freeze dryer? A Guide to Capacity, Performance & Features



















