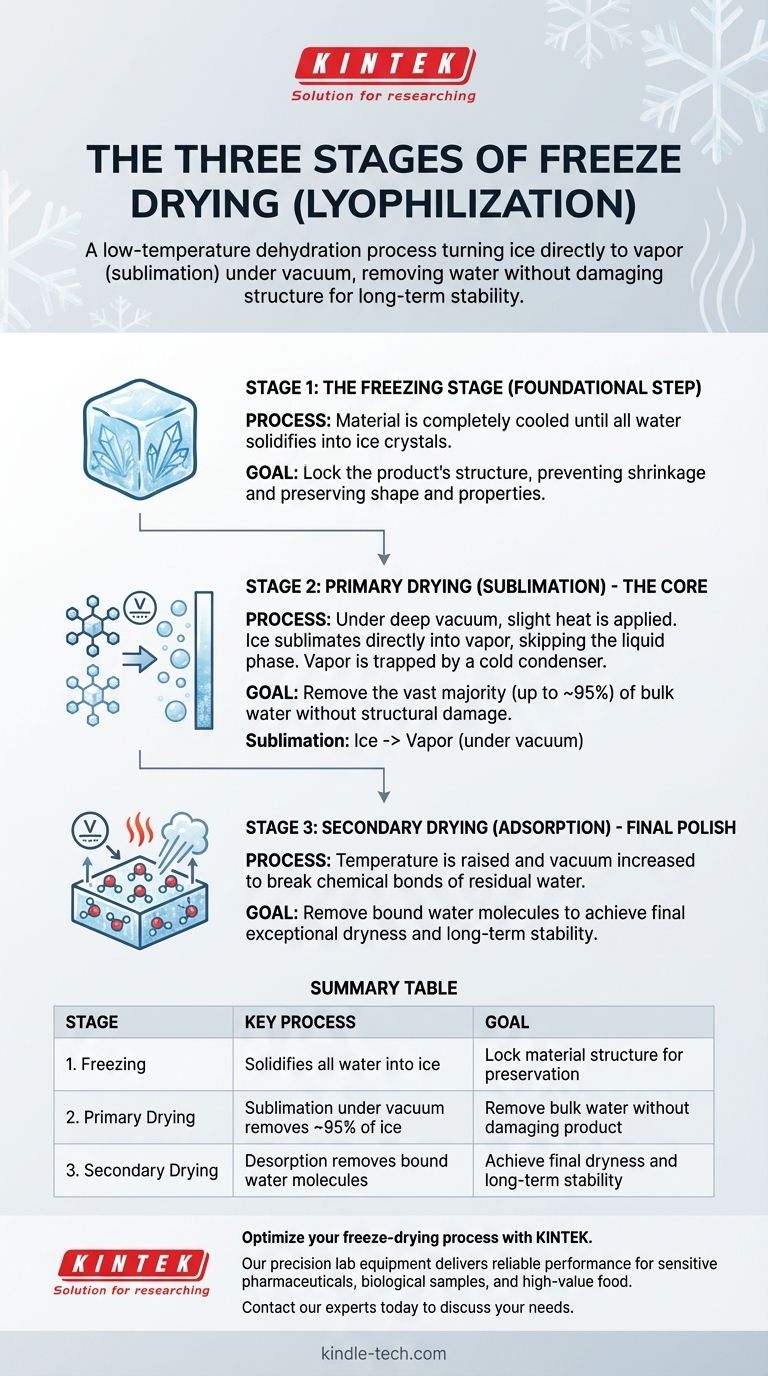
The three stages of freeze drying are the Freezing Stage, the Primary Drying Stage, and the Secondary Drying Stage. This low-temperature dehydration process, also known as lyophilization, first freezes the material completely, then uses a vacuum to turn the solid ice directly into a vapor, and finally removes any remaining bound water molecules to ensure long-term stability.
Freeze drying is a preservation technique designed to remove water without damaging a material's original structure. The core principle is sublimation—turning ice directly into vapor under a vacuum, which bypasses the destructive liquid phase entirely.

The Foundational Step: The Freezing Stage
The entire process depends on properly preparing the material by freezing it. This initial step locks the product's structure in place, which is critical for preserving its shape and properties.
### Why Freezing Comes First
Before water can be removed, it must be converted into a solid state (ice). Freezing prevents the material from shrinking or changing its appearance during dehydration, which is a common issue with conventional heat-based drying methods.
### The Goal: Complete Solidification
The material must be cooled to a temperature where it is completely frozen. This ensures that all the water is locked in place as ice crystals, ready for the next phase.
The Core of the Process: Primary Drying (Sublimation)
This is the longest and most critical phase of freeze drying. Here, the frozen water is removed from the product while it remains in its solid, frozen state.
### Introducing a Vacuum
Once the material is frozen, it is placed under a deep vacuum, which significantly lowers the surrounding pressure. This low-pressure environment is the key that enables sublimation to occur.
### The Physics of Sublimation
With the pressure lowered, a small amount of heat is carefully added. This energy gives the ice molecules just enough energy to transform directly from a solid into a gas (water vapor), skipping the liquid phase completely. The vacuum helps to speed up this process.
### The Role of the Condenser
As the water vapor leaves the product, it is collected by an extremely cold surface inside the freeze dryer called a condenser. Here, the vapor instantly turns back into ice, effectively trapping it and keeping it away from the product.
### How Much Water Is Removed
Primary drying is highly effective, removing the vast majority of the water content from the material—typically up to 95%.
The Final Polish: Secondary Drying (Adsorption)
After sublimation, a small amount of water molecules remains bound to the material's surface. The final stage is designed to remove this residual moisture.
### Targeting Bound Water
This last bit of water is more difficult to remove than the free ice crystals. It is chemically bonded (adsorbed) to the product's molecules.
### Adjusting Temperature and Pressure
To break these bonds, the temperature is gradually raised and the vacuum is often increased. This gives the remaining water molecules enough energy to detach and be drawn away, leaving the final product exceptionally dry and stable.
Understanding the Trade-offs
While highly effective, the freeze-drying process is not without its challenges. Understanding its limitations is key to using it successfully.
### The Time Commitment
Freeze drying is a very slow and deliberate process. The primary drying phase, in particular, can take a significant amount of time to ensure all the ice can sublimate without damaging the product.
### The Risk of Structural Damage
Applying too much heat during the primary drying stage can be detrimental. If the heat is too high, it can overwhelm the sublimation process, causing parts of the product to melt and altering its fundamental structure.
Making the Right Choice for Your Goal
The precision of the freeze-drying process makes it ideal for sensitive materials where preservation of structure and activity is paramount.
- If your primary focus is preserving biological or pharmaceutical materials: Freeze drying is the gold standard for maintaining the integrity of drugs, vaccines, and research samples for long-term storage.
- If your primary focus is creating high-quality, shelf-stable food: This process preserves the original taste, nutrition, and shape of food far better than traditional dehydration.
Ultimately, mastering the three stages of freeze drying allows for the remarkable preservation of materials that would otherwise be perishable or unstable.
Summary Table:
| Stage | Key Process | Goal |
|---|---|---|
| 1. Freezing | Solidifies all water into ice | Lock material structure for preservation |
| 2. Primary Drying | Sublimation under vacuum removes ~95% of ice | Remove bulk water without damaging product |
| 3. Secondary Drying | Desorption removes bound water molecules | Achieve final dryness and long-term stability |
Optimize your freeze-drying process with KINTEK.
Our precision lab equipment and consumables are designed to deliver the reliable performance and control required for each critical stage of lyophilization. Whether you are preserving sensitive pharmaceuticals, biological samples, or high-value food products, KINTEK solutions help you achieve superior results and long-term stability.
Contact our experts today to discuss your specific laboratory needs and discover the right equipment for your application.
Visual Guide
Related Products
- Benchtop Laboratory Freeze Dryer for Lab Use
- Benchtop Laboratory Vacuum Freeze Dryer
- Laboratory Sterilizer Lab Autoclave Pulse Vacuum Lifting Sterilizer
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
- Laboratory Test Sieves and Sieving Machines
People Also Ask
- What role does a laboratory freeze dryer play in the synthesis of graphene-based electrocatalysts? Preserve 3D Structures
- Why is a laboratory vacuum freeze dryer essential for plant extracts? Preserve Bioactivity & Structure
- What is the function of a freeze dryer in the ice-templating process? Preserving Aligned Pore Scaffolds for LAGP
- What is the function of Freeze-thaw Equipment in Au-(PNiPAAm/PVA) hydrogel? Achieve High-Speed Photothermal Actuation
- Why is a freeze dryer preferred over thermal drying for Fe-ZTA cermets? Ensure Pure, Homogeneous Slurry Processing



















