In the context of modern heat treatment, quenching is broadly categorized into two primary types based on the cooling medium used: gas quenching and liquid quenching. While gas quenching uses inert gases like nitrogen or argon to cool a workpiece in a controlled environment, liquid quenching submerges the workpiece in a fluid, typically oil or water, for much more rapid cooling.
While the question of "two types" often points to the choice between a gas or a liquid medium, the more critical concept for any professional is understanding the three physical stages of heat transfer that occur during quenching, as this is what truly dictates the final properties and integrity of the material.

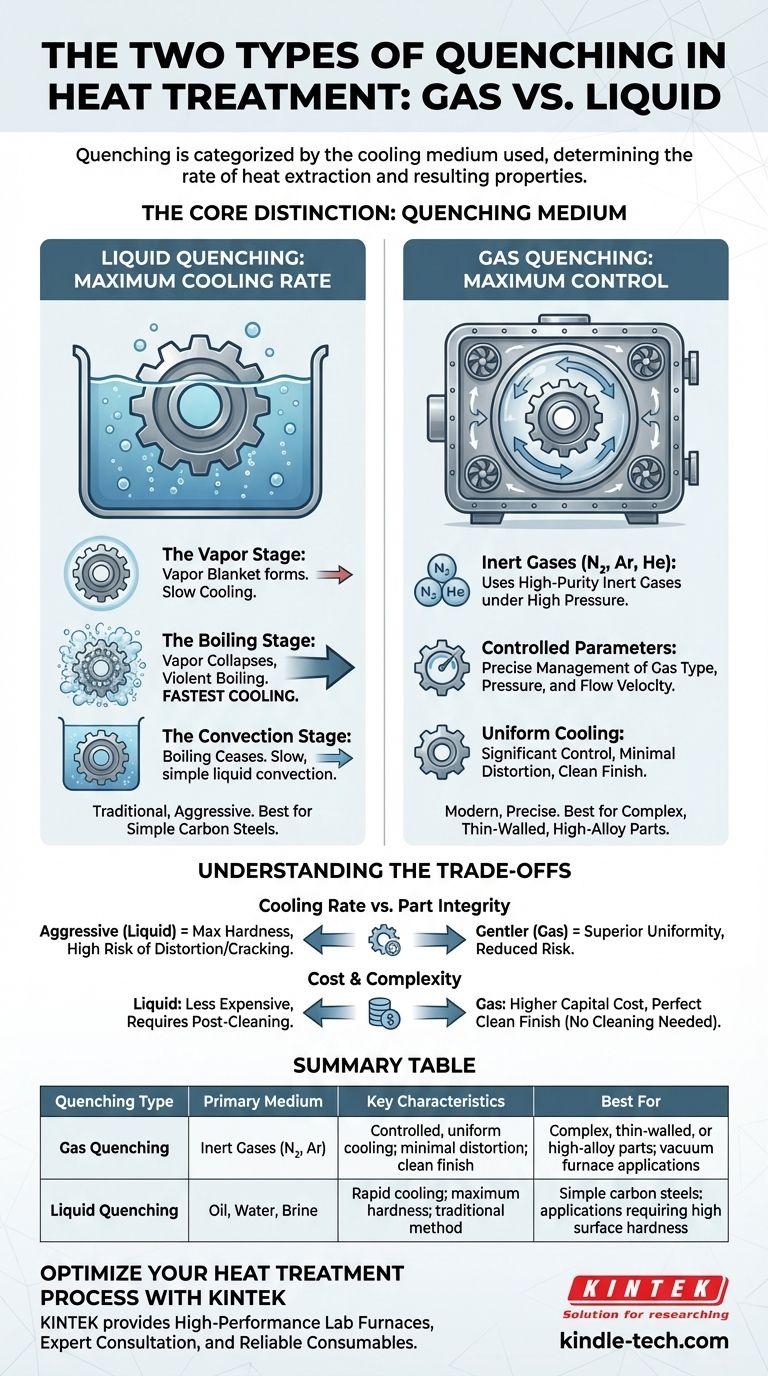
The Core Distinction: Quenching Medium
The choice of quenching medium is the most fundamental decision in the process. It directly controls the maximum rate of heat extraction, which in turn determines the resulting microstructure and mechanical properties of the steel.
Liquid Quenching: Maximum Cooling Rate
Liquid quenching is the traditional and most aggressive method of cooling. It involves submerging a hot component into a bath of liquid, most commonly water, brine, or specialized oils.
The process is defined by three distinct physical stages:
-
The Vapor Stage: Immediately upon immersion, the liquid touching the hot surface vaporizes, forming an insulating blanket of vapor around the part. Cooling is relatively slow in this stage as heat must radiate through this gas layer.
-
The Boiling Stage: As the part's surface cools, the vapor blanket becomes unstable and collapses. This initiates violent boiling, where heat is removed from the component at the fastest possible rate. This is the most critical phase for achieving hardness.
-
The Convection Stage: Once the surface temperature drops below the liquid's boiling point, boiling ceases. Cooling continues at a much slower rate through simple liquid convection, similar to a hot spoon cooling in a cup of coffee.
Gas Quenching: Maximum Control
Gas quenching is a more modern technique, typically performed within a vacuum furnace. After heating, the chamber is backfilled under high pressure with a high-purity, inert gas like nitrogen, argon, or helium to cool the part.
This method offers significantly more control than liquid quenching. By adjusting the gas type, pressure, and flow velocity, the cooling rate can be precisely managed. This makes it ideal for sensitive, complex, or thin-walled parts where distortion or cracking is a primary concern.
Beyond Two Types: A Spectrum of Techniques
The categories of "gas" and "liquid" are high-level starting points. In practice, metallurgists use numerous specialized techniques that modify these basic processes to achieve specific outcomes.
Why So Many Methods?
These specialized methods are not entirely new types, but rather precise applications or interruptions of the quenching process. They are designed to manipulate the cooling curve to control stress, reduce distortion, and achieve unique combinations of properties within a single component.
Example: Interrupted Quenching
In an interrupted quench, a part is quenched in an aggressive liquid (like salt or oil) just long enough to pass the critical "nose" of the cooling curve but is removed before it cools completely. It is then allowed to air cool slowly. This prevents the formation of brittle structures that can form at lower temperatures, dramatically reducing internal stress and the risk of cracking.
Example: Selective Quenching
Selective quenching is used to harden only specific areas of a component. This can be done by immersing only a portion of the part, such as the teeth of a gear, or by using targeted spray quenching. This leaves the core or other sections of the part softer and tougher, creating a component with superior overall performance.
Understanding the Trade-offs
Choosing a quenching process is a balancing act. The ideal choice depends on the steel alloy, the part's geometry, and the desired final properties.
Cooling Rate vs. Part Integrity
The central trade-off is between cooling speed and mechanical integrity. An aggressive quench (e.g., in water) provides the fast cooling needed to achieve maximum hardness in low-alloy steels but carries a high risk of distortion and cracking. A slower quench (e.g., high-pressure gas) is much gentler on the part but may be insufficient to fully harden less-receptive alloys.
Cost, Complexity, and Finish
Liquid quenching systems are generally less expensive to implement but often result in parts that require post-process cleaning to remove residue. Gas quenching, especially high-pressure gas quenching (HPGQ), demands sophisticated and expensive vacuum furnace equipment but produces perfectly clean, bright parts with no need for secondary cleaning operations.
How to Choose the Right Quenching Approach
Your final choice should be dictated by the engineering goal for the component.
- If your primary focus is achieving maximum hardness in simple carbon steels: An aggressive liquid quench using water or brine is often the most effective and economical path.
- If your primary focus is minimizing distortion in complex or high-value alloy parts: Controlled gas quenching provides superior uniformity and significantly reduces the risk of scrapping the part.
- If your primary focus is creating a dual-property component (e.g., a hard surface with a tough core): Advanced techniques like selective or interrupted quenching are necessary to manipulate the material's final structure.
Understanding these principles moves you from simply choosing a method to intentionally engineering the final properties of your material.
Summary Table:
| Quenching Type | Primary Medium | Key Characteristics | Best For |
|---|---|---|---|
| Gas Quenching | Inert Gases (N₂, Ar) | Controlled, uniform cooling; minimal distortion; clean finish | Complex, thin-walled, or high-alloy parts; vacuum furnace applications |
| Liquid Quenching | Oil, Water, Brine | Rapid cooling; maximum hardness; traditional method | Simple carbon steels; applications requiring high surface hardness |
Optimize Your Heat Treatment Process with KINTEK
Choosing the right quenching method is critical to achieving the desired hardness, minimizing distortion, and ensuring the integrity of your laboratory components. Whether you need the rapid cooling of liquid quenching for maximum hardness or the precise control of gas quenching for complex geometries, KINTEK has the expertise and equipment to support your goals.
We provide:
- High-performance lab furnaces suitable for both gas and liquid quenching processes.
- Expert consultation to help you select the right technique for your specific alloy and application.
- Reliable consumables and ongoing support to keep your heat treatment operations running smoothly.
Ready to enhance your material properties and achieve consistent results? Contact our team today to discuss your laboratory's quenching needs and discover how KINTEK's solutions can drive your success.
Visual Guide
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering Brazing Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
- Graphite Vacuum Furnace High Thermal Conductivity Film Graphitization Furnace
People Also Ask
- How to vacuum out a furnace? A Step-by-Step Guide to Safe DIY Maintenance
- What is vacuum furnace high temperature? Unlock the Range for Your Material Processing
- What are the advantages of vacuum hardening? Achieve Superior Precision and Cleanliness for Critical Components
- What does a vacuum furnace do? Achieve High-Purity Heat Treatment for Superior Components
- What is the standard thickness of plating? Optimize Durability, Corrosion & Cost



















