In biomass pyrolysis, the most common catalysts are silicon and zeolite-based, materials adapted from the petrochemical industry. However, these conventional catalysts face significant challenges because the large, complex polymers in biomass are fundamentally different from smaller petrochemical molecules. This mismatch in size and structure limits their effectiveness and drives the search for more advanced catalytic solutions.
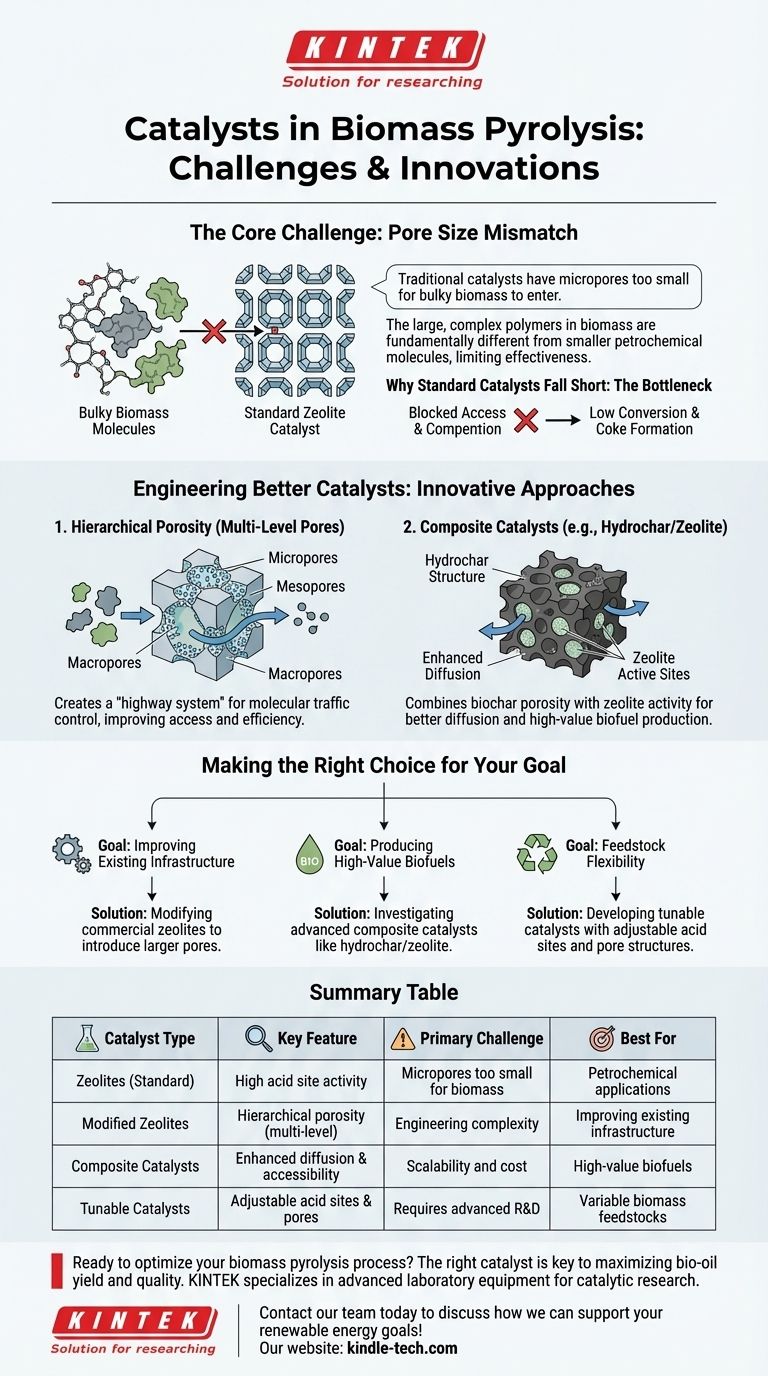
The core challenge in catalytic biomass pyrolysis is a physical mismatch: traditional catalysts have micropores too small for bulky biomass molecules to enter. The solution lies not just in chemical activity, but in re-engineering the catalyst's physical structure to improve molecular access and efficiency.

The Fundamental Role of a Catalyst
A catalyst's job is to steer chemical reactions toward a desired outcome. In pyrolysis, it directs the thermal decomposition of biomass to maximize the yield of valuable products like bio-oil and minimize unwanted byproducts.
Promoting Specific Reactions
Without a catalyst, pyrolysis is an uncontrolled thermal breakdown. A catalyst provides a surface with specific chemical properties that promote certain reactions, such as cracking long-chain molecules into shorter, more useful ones.
The Importance of Acid Sites
For biomass, the key catalytic function involves breaking down resilient carbon-carbon (C-C) and carbon-oxygen (C-O) bonds. This is achieved at specific acid sites on the catalyst's surface, which facilitate the cleavage of these bonds and the deoxygenation of the biomass vapors.
The Challenge: Why Standard Catalysts Fall Short
While effective in oil refining, commercial catalysts like zeolites struggle when applied directly to biomass. The issue is less about their chemical nature and more about their physical structure.
The "Pore Size" Bottleneck
Standard zeolites possess a network of extremely narrow micropores. While ideal for small petrochemical molecules, these pores are often too small for the bulky natural polymers and derived compounds from biomass, such as cellulose and lignin, to enter.
Blocked Access and Inefficiency
This size exclusion prevents the large molecules from reaching the internal acid sites where the conversion reactions occur. As a result, much of the catalytic potential is wasted, leading to lower conversion rates and the formation of undesirable coke on the catalyst's exterior surface.
Engineering Better Catalysts for Biomass
Addressing the limitations of standard catalysts requires innovative approaches that focus on improving molecular transport and access. The goal is to create a structure that accommodates the unique properties of biomass.
Creating Multi-Level Porosity
A key strategy is to create a multidimensional or hierarchical structure within the catalyst. By introducing larger meso- and macropores alongside the traditional micropores, a more efficient "highway system" for molecules is formed.
This structure allows large biomass molecules to easily enter the catalyst and be broken down into smaller intermediates, which can then access the micropores for final conversion. This improves what is known as molecular traffic control.
The Promise of Composite Catalysts
Another advanced approach involves creating composite materials. For example, hydrochar/zeolite composites combine the porous structure of biochar with the high activity of zeolites.
This design facilitates better diffusion of molecules into the catalyst, increasing the number of accessible active sites and making it highly suitable for producing advanced biofuels like biodiesel and bio-gasoline.
The Need for Tunable Catalysts
Biomass is not a uniform material; its composition varies widely between wood, agricultural waste, and algae. This variability demands tunable catalysts that can be adjusted to favor specific reactions, allowing producers to target desirable compounds based on the specific feedstock being used.
Making the Right Choice for Your Goal
The optimal catalytic strategy depends entirely on your specific objective, feedstock, and technological readiness.
- If your primary focus is improving existing infrastructure: Modifying commercial zeolites to introduce secondary, larger pores is the most direct path to better performance with biomass.
- If your primary focus is producing high-value biofuels: Investigating advanced composite catalysts like hydrochar/zeolite is critical for achieving the necessary conversion efficiency.
- If your primary focus is feedstock flexibility: Prioritize the development of tunable catalysts whose acid sites and pore structures can be adapted to different types of biomass.
Ultimately, unlocking the full potential of biomass as a renewable resource depends on designing catalysts that are structurally and chemically harmonized with its unique complexity.
Summary Table:
| Catalyst Type | Key Feature | Primary Challenge | Best For |
|---|---|---|---|
| Zeolites (Standard) | High acid site activity | Micropores too small for biomass molecules | Petrochemical applications |
| Modified Zeolites | Hierarchical porosity (multi-level pores) | Engineering complexity | Improving existing infrastructure |
| Composite Catalysts (e.g., Hydrochar/Zeolite) | Enhanced diffusion & accessibility | Scalability and cost | Producing high-value biofuels (biodiesel, bio-gasoline) |
| Tunable Catalysts | Adjustable acid sites & pore structures | Requires advanced R&D | Handling variable biomass feedstocks |
Ready to optimize your biomass pyrolysis process? The right catalyst is key to maximizing bio-oil yield and quality. KINTEK specializes in advanced laboratory equipment and consumables for catalytic research and biofuel development. Our experts can help you select the right tools to test and scale your catalytic solutions. Contact our team today to discuss how we can support your renewable energy goals!
Visual Guide
Related Products
- Custom PTFE Teflon Parts Manufacturer for PTFE Mesh F4 Sieve
- Molybdenum Disilicide (MoSi2) Thermal Elements Electric Furnace Heating Element
- Custom PTFE Teflon Parts Manufacturer for Magnetic Stirring Bar
- Custom PTFE Teflon Parts Manufacturer for PTFE Measuring Cylinder 10/50/100ml
- Automatic Laboratory Heat Press Machine
People Also Ask
- What are the low cost catalysts for pyrolysis? Optimize Your Pyrolysis Process with Affordable Catalysts
- How does a one-zone tubular furnace influence SiC coatings? Master CVD Precision & Material Hardness
- What is the effect of catalyst in pyrolysis? Upgrading Bio-Oil for Higher-Value Fuels
- Why is a quartz tube furnace utilized in the thermal oxidation of MnCr2O4 coatings? Unlock Precise Selective Oxidation
- Why is a high-temperature tube furnace necessary for Pt/SiC-C catalyst? Ensure Precision Synthesis & Metal Dispersion
- What is a vertical diffusion furnace? Achieve Superior Wafer Processing for Semiconductor Manufacturing
- What technical advantages do quartz tube reactors offer for SCR denitration? Eliminate Wall Effects for Pure Data
- What is the critical role of the tube sublimation furnace in CVT? Pure ZnS Crystal Prep