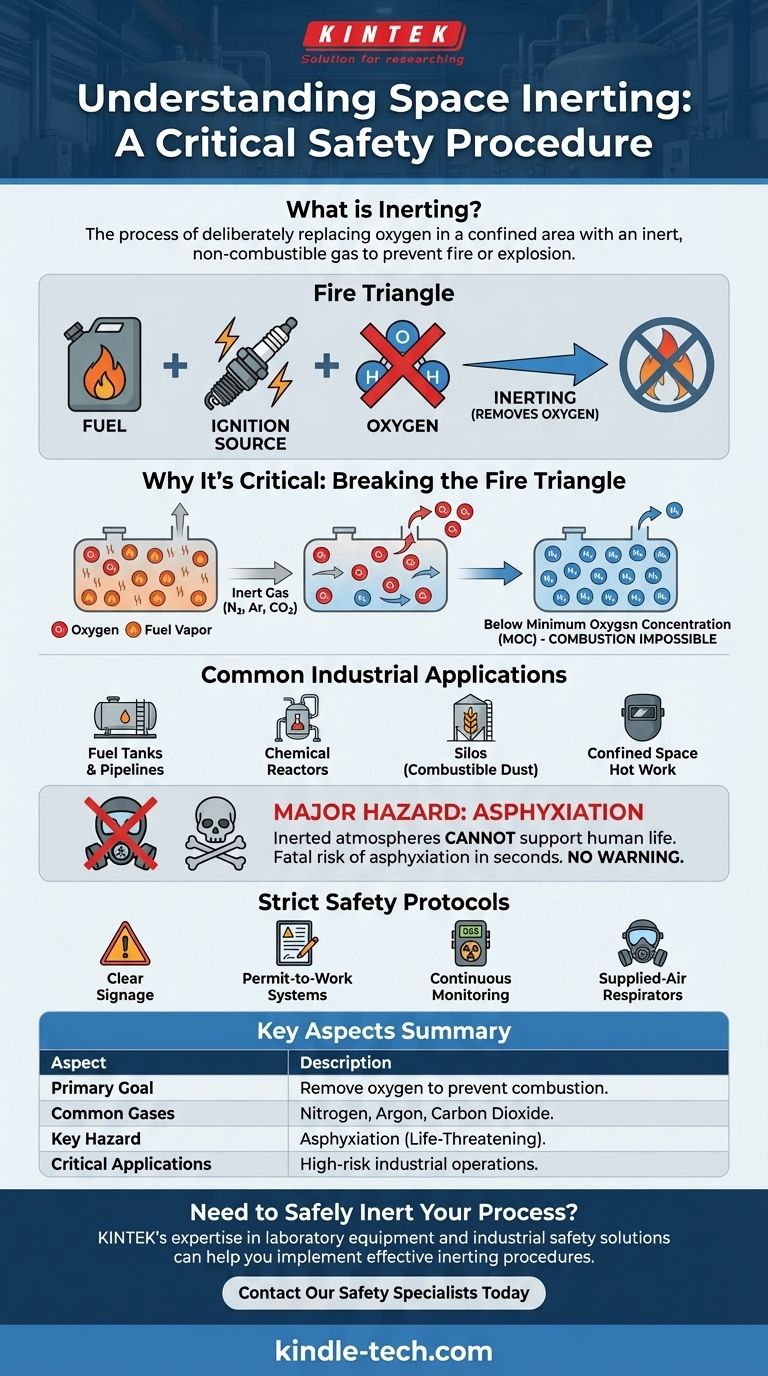
In its simplest terms, inerting a space is the process of deliberately replacing the oxygen in a confined area with an inert, non-combustible gas. This is a specialized form of purging designed to make the atmosphere incapable of supporting a fire or explosion, even if fuel and an ignition source are present.
The core purpose of inerting is not to clean a space, but to proactively remove one critical element of the fire triangle—oxygen—thereby eliminating the possibility of combustion.

Why Inerting is a Critical Safety Procedure
To understand inerting, you must first understand the basic principle of fire. Any fire or explosion requires three components, often called the "fire triangle."
The Fire Triangle Explained
The three essential elements for combustion are fuel (like a flammable vapor), an ignition source (like a spark or static electricity), and oxygen. If you remove any one of these three elements, a fire cannot start or sustain itself.
Inerting is a safety control that focuses exclusively on eliminating the oxygen component from this equation.
Creating a Non-Combustible Atmosphere
By flooding a space with an inert gas like nitrogen, argon, or carbon dioxide, the oxygen concentration is diluted and displaced. The goal is to lower the oxygen level below the Minimum Oxygen Concentration (MOC) required for the specific fuel to ignite.
Once the atmosphere is inerted, a spark or other ignition source will fail to start a fire because there is not enough oxygen to support the chemical reaction.
Common Industrial Applications
This procedure is critical in high-hazard environments where flammable materials are present. Common applications include:
- Fuel tanks and pipelines during maintenance
- Chemical reactors processing volatile substances
- Silos containing combustible dust, like grain or coal
- Confined spaces where welding or other "hot work" must be performed
Understanding the Primary Risks
While highly effective at preventing fires, the process of inerting introduces a different but equally severe hazard. Understanding this trade-off is non-negotiable.
The Life-Threatening Hazard of Asphyxiation
An atmosphere that cannot support a fire also cannot support human life. The primary danger of an inerted space is asphyxiation, which can occur in seconds and without warning.
The human body cannot detect a lack of oxygen. Entering an inerted space without proper breathing apparatus is fatal.
The Need for Strict Safety Protocols
Because of the asphyxiation risk, inerting requires rigorous safety procedures. These include:
- Clear Signage: Warning personnel that the space is inerted and unsafe to enter.
- Permit-to-Work Systems: Requiring formal authorization for entry.
- Continuous Monitoring: Using gas detectors to verify oxygen levels before entry is permitted.
- Supplied-Air Respirators: Mandating specialized breathing equipment for anyone entering the space.
Making the Right Choice for Your Goal
Applying this knowledge depends entirely on your objective, whether it's designing a safe process or ensuring personal safety.
- If your primary focus is process safety: The goal is to reduce and maintain the oxygen concentration below the MOC for the specific flammable material you are handling.
- If your primary focus is personnel safety: The non-negotiable rule is that an inerted space is an immediate danger to life and must only be entered with supplied-air breathing apparatus under a strict permit system.
- If your primary focus is operational planning: The choice of inert gas (e.g., nitrogen vs. argon) will depend on factors like cost, availability, and the density required for effective displacement of the existing atmosphere.
Inerting is an essential engineering control that makes high-risk industrial operations possible, but it must be managed with an absolute understanding of its inherent dangers.
Summary Table:
| Key Aspect | Description |
|---|---|
| Primary Goal | Remove oxygen to prevent combustion by breaking the fire triangle. |
| Common Gases Used | Nitrogen, Argon, Carbon Dioxide. |
| Key Safety Hazard | Asphyxiation - the inerted atmosphere cannot support human life. |
| Critical Applications | Fuel tank maintenance, chemical reactors, silos with combustible dust, hot work in confined spaces. |
Need to safely inert a process or storage space?
KINTEK's expertise in laboratory equipment and industrial safety solutions can help you implement effective inerting procedures. We provide the reliable equipment and consumables necessary for creating and monitoring safe, non-combustible atmospheres.
Contact our safety specialists today to discuss your specific application and ensure your operations are protected from fire and explosion risks.
Visual Guide
Related Products
- Laboratory High Pressure Horizontal Autoclave Steam Sterilizer for Lab Use
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Copper Foam
- Platinum Auxiliary Electrode for Laboratory Use
- 20L Heating Chilling Circulator Cooling Water Bath Circulator for High and Low Temperature Constant Temperature Reaction
People Also Ask
- Why is a high-temperature atmosphere furnace required for 20Cr-25Ni-Nb stainless steel? Expert Heat Treatment Guide
- How does a controlled atmosphere work? A Guide to Precision Gas Control
- What is the working mechanism of a high-purity hydrogen atmosphere furnace? Master Tungsten Sintering Efficiency
- Why is atmosphere-protected heating equipment necessary for Pyr-IHF? Achieve Precision in Material Synthesis
- Why are atmosphere control and temperature precision critical for single-crystal cathode synthesis?
- What is the function of a tube atmosphere furnace in MLM? Optimize Your CNT/Cu Composite Production
- How do laboratory atmosphere furnaces facilitate the sintering of Ti2AlC foams? Achieve High-Strength Porous Ceramics
- What is the cheapest inert gas? Argon is the Cost-Effective Choice for True Inertness

















