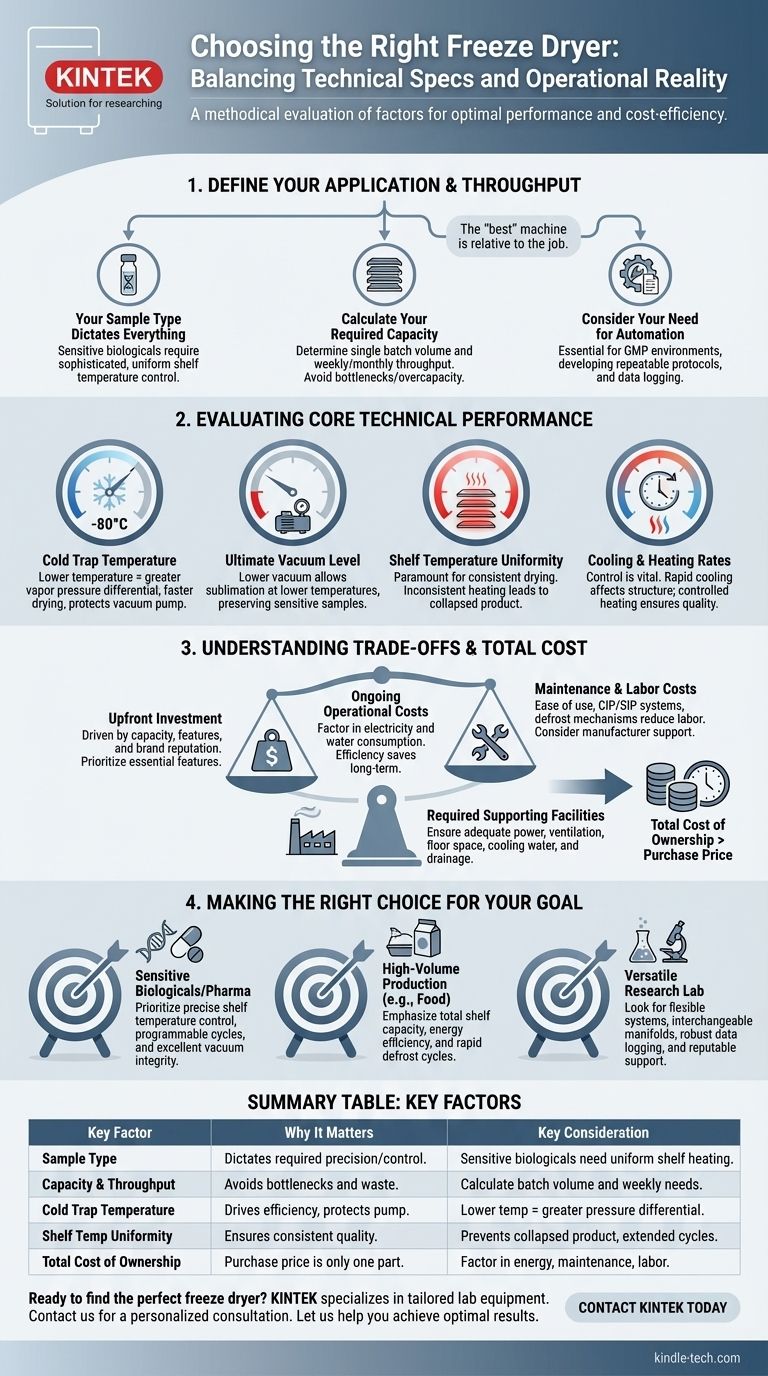
Choosing the right freeze dryer requires a methodical evaluation that goes beyond a simple features checklist. You must balance the core technical specifications required for your specific application against the long-term operational costs and workflow integration. Key performance indicators like cold trap temperature, ultimate vacuum, and shelf temperature uniformity are critical, but they must be weighed against factors like sample capacity, batch duration, and total cost of ownership.
The ideal freeze dryer is not the one with the highest specifications, but the one whose performance, capacity, and operational costs are precisely aligned with your specific samples, throughput requirements, and budget. Prioritizing essential features over unnecessary complexity is the key to making a sound investment.

First, Define Your Application and Throughput
Before comparing models, you must have a clear picture of your exact needs. The "best" machine is entirely relative to the job it needs to perform.
Your Sample Type Dictates Everything
The physical and chemical properties of your samples are the most important starting point. Sensitive biologicals or pharmaceuticals require a level of precision that other materials may not.
If you are working with delicate samples, a system with sophisticated and uniform shelf temperature control is non-negotiable. This ensures the product remains below its critical collapse temperature throughout the primary drying phase.
Calculate Your Required Capacity
Consider both the volume of a single batch and your required throughput over a week or month. This will determine the necessary shelf space or number of ports.
Underestimating your capacity needs leads to production bottlenecks, while overestimating leads to wasted capital and higher energy consumption per cycle.
Consider Your Need for Automation
Modern freeze dryers offer a wide range of automation and data logging capabilities. These features are essential for regulated environments (like GMP manufacturing) or for developing repeatable scientific protocols.
Basic operations may not require this level of control, but for process validation and consistency, it is a critical feature.
Evaluating Core Technical Performance
Once your application is defined, you can assess the technical specifications of a potential machine. These four indicators are the most critical for performance.
Cold Trap Temperature
The cold trap, or condenser, is responsible for trapping the water vapor sublimated from your product. Its surface must be significantly colder than the product's eutectic or glass transition temperature to work efficiently.
A lower cold trap temperature creates a greater vapor pressure differential, which speeds up the drying process and protects the vacuum pump from moisture.
Ultimate Vacuum Level
The vacuum pump removes air from the system, lowering the pressure to a point where ice can turn directly into vapor (sublimation) at a low temperature.
A lower ultimate vacuum capability allows for drying at lower temperatures, which is crucial for preserving the structure of extremely sensitive samples.
Shelf Temperature Uniformity
For any product dried on shelves, temperature uniformity across all shelves—and within a single shelf—is paramount.
Inconsistent heating can lead to some parts of the batch drying faster than others, resulting in collapsed product, extended cycle times, or inconsistent final moisture content.
Cooling and Heating Rates
The ability to control the rate of cooling and heating is vital for process optimization. Rapid cooling can affect the ice crystal structure of the sample, while controlled heating during primary and secondary drying is essential for efficiency and product quality.
Understanding the Trade-offs and Total Cost
A freeze dryer is a significant capital investment, and the purchase price is only one part of the equation.
The Upfront Investment
The price of a freeze dryer is driven primarily by its capacity, features, and the manufacturer's reputation. A larger, more sophisticated machine from a leading brand will have a higher initial cost.
It is crucial to prioritize features that are essential for your specific needs rather than opting for unnecessary and expensive add-ons.
Ongoing Operational Costs
Factor in the consumption of electricity and water (for water-cooled models). A more efficient machine may have a higher purchase price but could save you a significant amount in utility costs over its lifespan.
Maintenance and Labor Costs
Consider the ease of use and maintenance requirements. Features like clean-in-place (CIP) or steam-in-place (SIP) systems and simple defrost mechanisms can dramatically reduce the labor required to operate and maintain the unit.
The manufacturer's reputation for service and support is also a critical factor in minimizing potential downtime.
Required Supporting Facilities
Ensure you have the necessary infrastructure to support the machine. This includes adequate electrical supply, ventilation, floor space, and, for larger units, access to cooling water or drainage.
Making the Right Choice for Your Goal
To select the best freeze dryer, align your choice with your primary operational driver.
- If your primary focus is on sensitive biologicals or pharmaceuticals: Prioritize a machine with precise shelf temperature control, programmable cycles for process optimization, and excellent vacuum integrity.
- If your primary focus is on high-volume production (e.g., food): Emphasize total shelf capacity, energy efficiency, and rapid defrost cycles to maximize throughput and minimize the operational cost per batch.
- If your primary focus is on a versatile research lab environment: Look for a flexible system with interchangeable manifolds and flask options, robust data logging for experiments, and a reputable manufacturer for reliable support.
A thorough assessment of both your technical needs and the long-term operational realities will ensure your freeze dryer becomes a reliable asset, not a costly liability.
Summary Table:
| Key Factor | Why It Matters | Key Consideration |
|---|---|---|
| Sample Type | Dictates required precision and temperature control. | Sensitive biologicals need uniform shelf heating. |
| Capacity & Throughput | Avoids bottlenecks and wasted resources. | Calculate batch volume and weekly/monthly needs. |
| Cold Trap Temperature | Drives drying efficiency and protects the vacuum pump. | Lower temperature creates a greater vapor pressure differential. |
| Shelf Temperature Uniformity | Ensures consistent drying and product quality. | Prevents collapsed product and extended cycle times. |
| Total Cost of Ownership | Purchase price is just one part of the investment. | Factor in energy consumption, maintenance, and labor costs. |
Ready to find the perfect freeze dryer for your laboratory?
Choosing the right equipment is critical for your research, production, or quality control. The experts at KINTEK specialize in providing lab equipment and consumables tailored to your specific needs. We can help you navigate the technical specifications and operational costs to ensure your freeze dryer is a sound investment that enhances your workflow.
Contact KINTEK today for a personalized consultation. Let us help you achieve optimal results with equipment that is precisely aligned with your samples, throughput, and budget.
Visual Guide
Related Products
- Benchtop Laboratory Freeze Dryer for Lab Use
- Benchtop Laboratory Vacuum Freeze Dryer
- Laboratory Sterilizer Lab Autoclave Pulse Vacuum Lifting Sterilizer
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
- Laboratory Test Sieves and Sieving Machines
People Also Ask
- Why is a laboratory freeze-drying system essential for fermentation biomass? Preserve Sample Integrity for Analysis
- What is the function of Freeze-thaw Equipment in Au-(PNiPAAm/PVA) hydrogel? Achieve High-Speed Photothermal Actuation
- Why is a laboratory vacuum freeze dryer essential for plant extracts? Preserve Bioactivity & Structure
- Why is a freeze dryer preferred for drying nickel nanoparticle precursors? Prevent Hard Agglomeration Now
- What is the function of a freeze dryer in the ice-templating process? Preserving Aligned Pore Scaffolds for LAGP



















