At its core, sintering is a high-temperature process that transforms a fragile, compacted powder into a dense, strong, and solid ceramic object. By applying heat just below the material's melting point, individual ceramic particles fuse, eliminating the voids between them and causing the entire part to shrink and increase in density. This atomic-level bonding is what gives sintered ceramics their exceptional mechanical and physical properties.
Sintering is not simply baking; it is a controlled microstructural engineering process. Its fundamental purpose is to eliminate porosity by enabling atoms to diffuse across particle boundaries, thereby transforming a weak powder compact into a robust, high-performance solid.

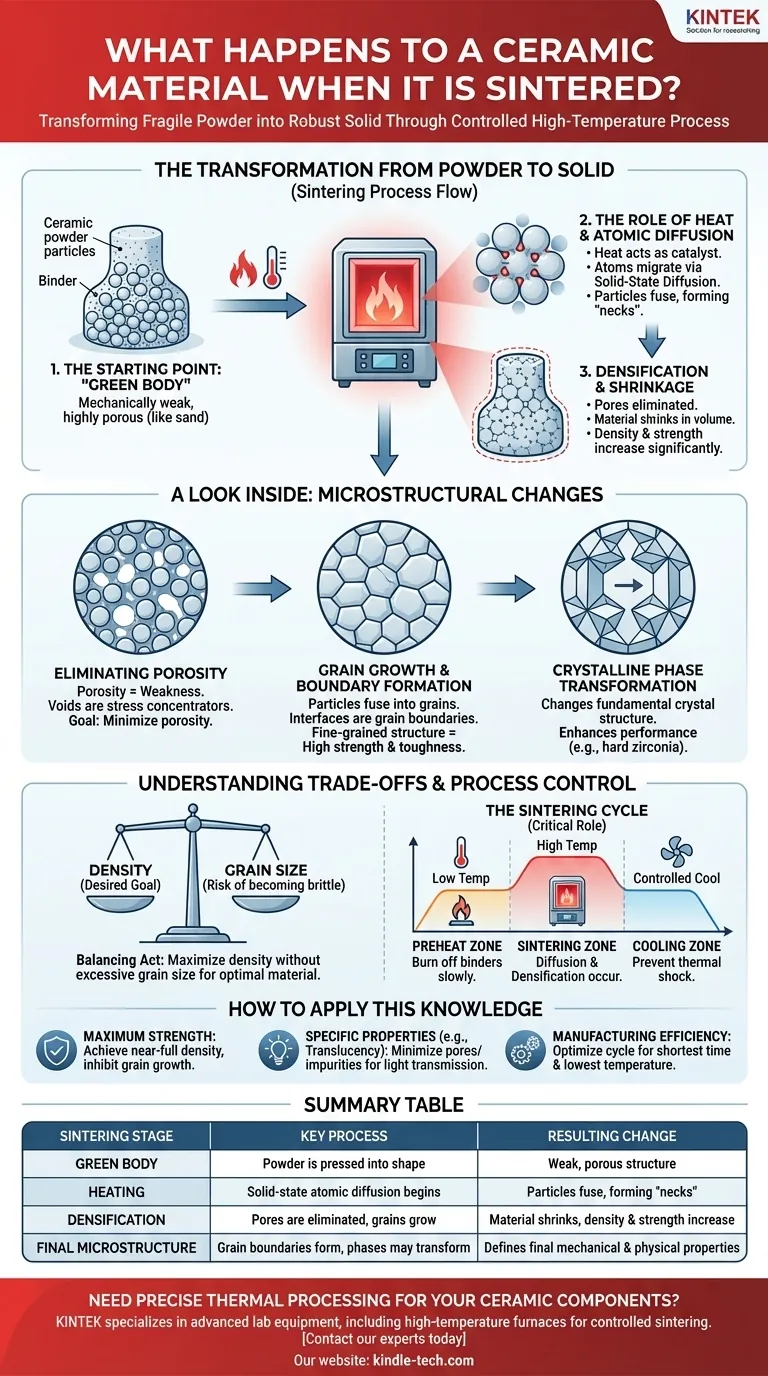
The Transformation from Powder to Solid
Sintering is the critical step that converts a shaped but fragile material into a functional, finished component. The process is a journey of atomic movement and structural consolidation.
The Starting Point: The "Green Body"
Before sintering, the ceramic exists as a "green body." This is formed by pressing ceramic powder, often mixed with a binder, into the desired shape.
The green body has shape and some handling strength, but it is mechanically weak and highly porous, much like a tightly packed pile of sand.
The Role of Heat and Atomic Diffusion
Heat is the catalyst for sintering. As the green body is heated in a kiln, the atoms in the ceramic particles gain enough energy to move.
This movement, known as solid-state diffusion, allows atoms to migrate from one particle to another at their points of contact. The particles begin to fuse, forming "necks" that grow over time.
Densification and Shrinkage
As the particles fuse and rearrange to form a denser structure, the pores between them are gradually eliminated.
This elimination of void space causes the entire component to shrink in volume. This shrinkage is a direct and visible indicator of successful densification, resulting in a significantly harder and stronger final part.
A Look Inside: Microstructural Changes
The remarkable properties of a sintered ceramic are a direct result of changes that occur on a microscopic level. The final microstructure dictates the material's performance.
Eliminating Porosity
Porosity is the primary source of weakness in an unsintered ceramic. These voids act as stress concentrators, where cracks can easily initiate and propagate under load.
The main objective of sintering is to reduce this porosity to a minimum, creating a dense material that can effectively resist mechanical failure.
Grain Growth and Boundary Formation
As the original particles fuse, they form larger, interlocking crystals known as grains. The interfaces where these different grains meet are called grain boundaries.
The final size and shape of these grains are critical. Generally, a fine-grained structure is desirable for high strength and toughness.
Crystalline Phase Transformation
In some advanced ceramics, sintering does more than just densify the material. It can also trigger a change in the material's fundamental crystal structure.
For example, zirconia is transformed from a weaker monoclinic state to an exceptionally hard and tough polytetragonal crystalline state during sintering, dramatically enhancing its performance for demanding applications.
Understanding the Trade-offs and Process Control
Sintering is a delicate balance. Achieving the desired outcome requires precise control over the process variables, as each presents a potential trade-off.
The Balance Between Density and Grain Size
The primary goal is to achieve maximum density, but this requires significant time at high temperatures. Unfortunately, these same conditions also promote grain growth.
If grains grow too large, the material can become more brittle, even if it is fully dense. The ideal process achieves high density while keeping the grain size small and uniform.
The Risk of Incomplete Sintering
Applying insufficient heat or time results in incomplete sintering. The part will retain significant residual porosity, making it weak, unreliable, and often unusable for its intended purpose.
The Critical Role of the Sintering Cycle
The heating and cooling process, or "sintering cycle," must be meticulously controlled. A typical cycle in a tunnel kiln includes:
- Preheat Zone: A lower-temperature stage to slowly burn off any binders or lubricants from the green body.
- Sintering Zone: The high-temperature hold where diffusion and densification occur.
- Cooling Zone: A controlled cooling phase to prevent thermal shock and cracking.
How to Apply This Knowledge
Understanding the principles of sintering allows you to connect process decisions to final material outcomes.
- If your primary focus is maximum strength and durability: The goal is to achieve near-full density while implementing strategies to inhibit excessive grain growth, such as optimizing temperature and hold times.
- If your primary focus is achieving specific properties (like translucency): The goal shifts to precise control over the final microstructure, minimizing any residual pores or impurities that could scatter light.
- If your primary focus is manufacturing efficiency: The goal is to optimize the sintering cycle for the shortest time and lowest temperature that still achieves the required density and properties.
Ultimately, mastering the sintering process is about precisely controlling atomic-level changes to build exceptional material performance from the ground up.
Summary Table:
| Sintering Stage | Key Process | Resulting Change |
|---|---|---|
| Green Body | Powder is pressed into shape | Weak, porous structure |
| Heating | Solid-state atomic diffusion begins | Particles fuse, forming 'necks' |
| Densification | Pores are eliminated, grains grow | Material shrinks, density & strength increase |
| Final Microstructure | Grain boundaries form, phases may transform | Defines final mechanical & physical properties |
Need precise thermal processing for your ceramic components? KINTEK specializes in advanced lab equipment, including high-temperature furnaces perfect for controlled sintering cycles. Our solutions help you achieve the perfect balance of density and grain size for superior material performance. Contact our experts today to discuss your specific laboratory sintering requirements!
Visual Guide
Related Products
- Vacuum Dental Porcelain Sintering Furnace
- Dental Porcelain Zirconia Sintering Ceramic Furnace Chairside with Transformer
- 600T Vacuum Induction Hot Press Furnace for Heat Treat and Sintering
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
- Vacuum Heat Treat and Sintering Furnace with 9MPa Air Pressure
People Also Ask
- What functions do high-purity alumina support rods serve in sCO2 experiments? Ensure High-Temp Material Integrity
- Why are alumina insulation discs used as sample supports in CCPN? Ensure Arcing-Free, Uniform Plasma Nitriding
- What catalytic effects do alumina ceramic surfaces have on biomass gasification? Boost Syngas Heating Value
- What industry is silicon carbide used in? Powering Semiconductor, Aerospace, and High-Temp Applications
- What temperature do you fire alumina? Achieve Optimal Density and Strength
- What is ceramic powder made of? A Guide to Advanced Ceramic Materials and Their Uses
- What is the specific heat of alumina? It's a range from 451 to 955 J/kg·K
- What determines the strength of ceramics? The surprising role of microscopic flaws in brittle failure



















