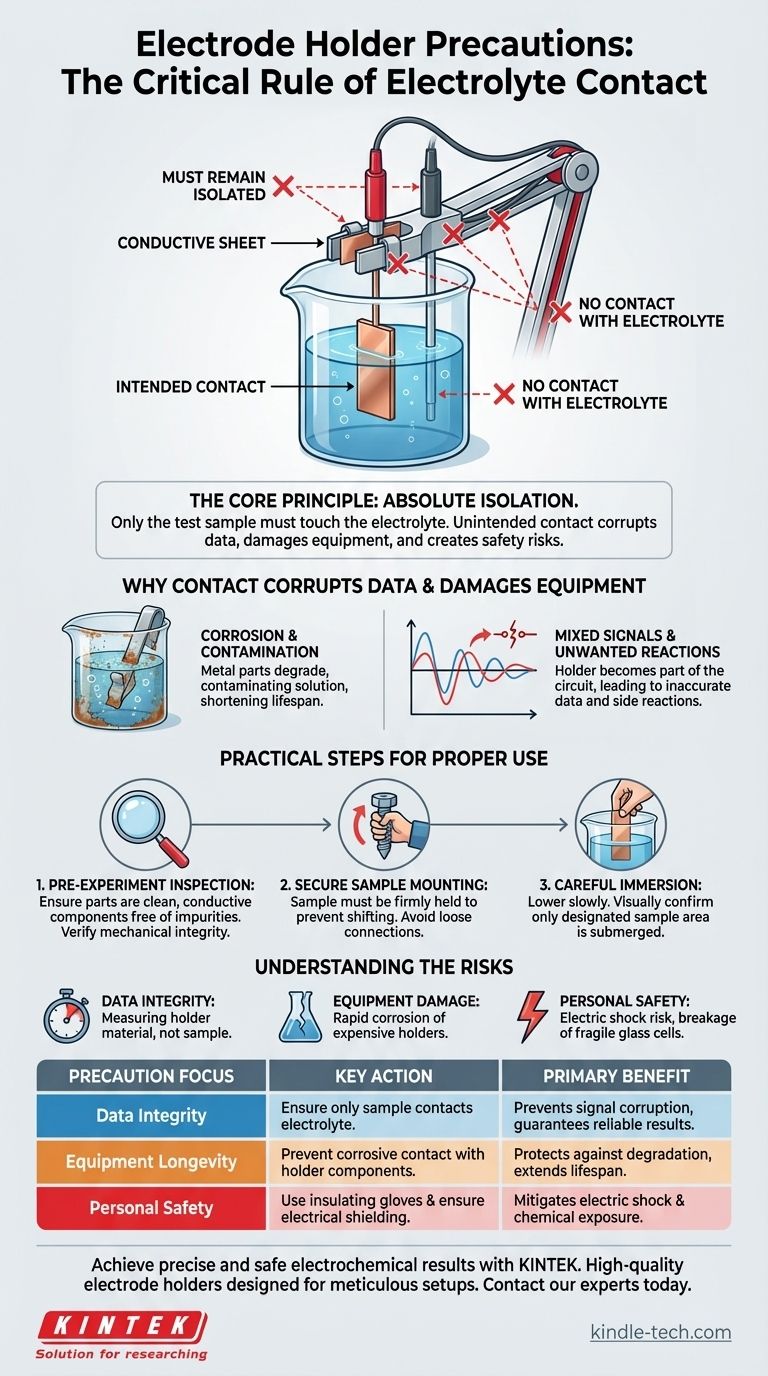
The single most critical precaution when using an electrode holder is to ensure that only the intended test sample makes contact with the electrolyte solution. All other parts of the holder, including the conductive sheet, clips, and electrode rod, must remain completely isolated from the electrolyte to guarantee the validity and safety of your experiment.
The core principle is one of absolute isolation. Any unintended contact between the holder and the electrolyte introduces a foreign variable, corrupting your data, potentially damaging the equipment, and creating safety risks.

The Core Principle: Isolating the Reaction
The entire purpose of an electrochemical setup is to measure reactions occurring at a specific surface—your test sample. Allowing other components to touch the electrolyte fundamentally breaks this controlled environment.
Why Contact Corrupts Data
When a metal part of the holder touches the electrolyte, it becomes an active part of the electrochemical circuit. This can lead to unwanted side reactions, current leakage, or measurement of the holder's properties instead of the sample's.
Your results will no longer reflect the behavior of your sample alone. They become a mixed signal, rendering the data inaccurate and unreliable.
The Consequence of Corrosion
Electrolyte solutions are often corrosive. Unintended contact can quickly degrade the metallic components of your electrode holder, leading to equipment failure and contaminating your solution with dissolved metals.
This not only shortens the lifespan of your equipment but also introduces impurities that can interfere with the very reaction you are trying to study.
Practical Steps for Proper Use
Meticulous preparation and handling are non-negotiable for achieving reliable results. Following a clear protocol prevents the most common sources of error.
Pre-Experiment Inspection
Before every use, ensure the conductive sheet and the sample surface are clean and free of any impurities. If the conductive components are dirty, they can be rinsed with deionized water.
Also, confirm that all mechanical parts, such as clips and fastening screws, are in good working order to hold the sample securely throughout the experiment.
Secure Sample Mounting
A loosely mounted sample can shift or fall during the experiment, leading to unintended contact between the holder and the electrolyte. Ensure the sample is held firmly in place before immersion.
Careful Immersion
Lower the mounted sample into the electrolyte solution slowly and deliberately. Visually confirm that only the designated area of the test sample is submerged and that the solution level is well below any part of the holder itself.
Understanding the Risks and Pitfalls
Failing to isolate the holder creates three primary categories of risk: data integrity, equipment damage, and personal safety.
Risk of Inaccurate Data
This is the most immediate scientific consequence. If your holder is made of stainless steel and it touches the solution, you may inadvertently measure the electrochemistry of steel instead of your intended material.
Risk of Equipment Damage
The materials used in the holder's clamps and rods are not always designed to withstand the specific chemistry of your electrolyte. This can lead to rapid corrosion, ruining a costly piece of equipment.
Risk of Electric Shock
To prevent electric shock, ensure any metal parts of the holder or stand are properly insulated. Always wear insulating gloves during operation and never handle electrodes or other components with wet hands.
Risk of Physical Damage
Many electrochemical cells are made of glass, which is fragile. Always handle these components gently to prevent breakage, which could lead to spills and exposure to hazardous chemicals.
Ensuring a Successful Experiment
Your experimental goal determines which precautions are most critical. Use this guide to focus your preparation.
- If your primary focus is data integrity: Your absolute priority is ensuring only the test sample's surface touches the electrolyte.
- If your primary focus is equipment longevity: Meticulous cleaning after use and preventing corrosive electrolyte contact are key.
- If your primary focus is personal safety: Always wear insulating gloves and ensure all electrical components are properly shielded from the solution.
Ultimately, a disciplined and careful setup is the foundation of trustworthy and repeatable scientific results.
Summary Table:
| Precaution Focus | Key Action | Primary Benefit |
|---|---|---|
| Data Integrity | Ensure only the test sample contacts the electrolyte. | Prevents signal corruption and guarantees reliable results. |
| Equipment Longevity | Prevent corrosive electrolyte contact with holder components. | Protects against degradation and extends equipment lifespan. |
| Personal Safety | Use insulating gloves and ensure proper electrical shielding. | Mitigates risks of electric shock and chemical exposure. |
Achieve precise and safe electrochemical results with the right equipment and expertise.
KINTEK specializes in high-quality lab equipment and consumables for all your electrochemical needs. Our durable electrode holders and accessories are designed to support meticulous experimental setups, helping you maintain the critical isolation required for valid data.
Let us help you enhance your lab's efficiency and safety. Contact our experts today to discuss your specific requirements and discover how our solutions can protect your experiments and your investment.
Visual Guide
Related Products
- Electrolytic Electrochemical Cell for Coating Evaluation
- Customizable PEM Electrolysis Cells for Diverse Research Applications
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- Custom PTFE Wafer Holders for Lab and Semiconductor Processing
- Button Battery Case for Battery Lab Applications
People Also Ask
- What type of electrode system is the coating evaluation electrolytic cell designed for? Unlock Precise Coating Analysis
- How is a high-precision electrolytic cell used to evaluate metal corrosion resistance? Validate DCT Results Accurately
- What are the advantages of a flat electrochemical cell for corrosion? Achieve Precise Pitting & Crevice Analysis
- What is the operating principle of a flat plate corrosion electrolytic cell? A Guide to Controlled Materials Testing
- How does a three-electrode electrolytic cell function? Precision Testing for 8620 Steel in Corrosive Environments














