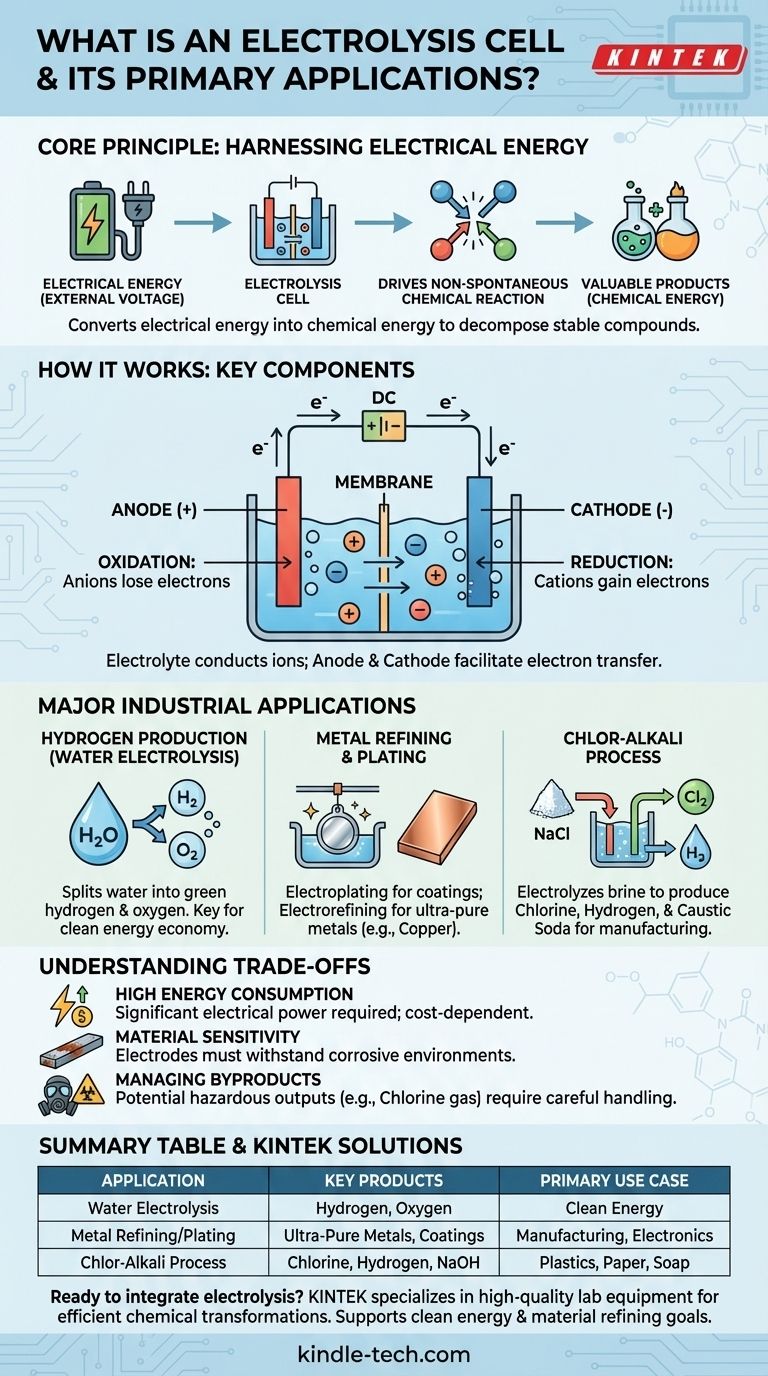
In essence, an electrolysis cell is a device that harnesses electrical energy to drive a chemical reaction that would not otherwise occur on its own. It works by passing an electric current through a substance, typically a solution containing ions, to decompose or transform it. Key industrial applications include producing hydrogen from water, refining metals like copper, and manufacturing essential chemicals like chlorine and sodium hydroxide.
An electrolysis cell fundamentally converts electrical energy into chemical energy. It overcomes the natural stability of compounds, using electricity as a tool to break them apart and create new, often more valuable, substances.

How an Electrolysis Cell Works
An electrolysis cell operates on the principle of electrolysis, which literally means "to break apart with electricity." This process is the opposite of what happens in a battery (a galvanic cell), which produces electricity from a spontaneous chemical reaction.
The Core Principle: Forcing a Reaction
Many valuable chemical compounds, like water (H₂O) or salt (NaCl), are very stable. They do not spontaneously break down into their constituent elements.
An electrolysis cell provides the necessary energy, in the form of an external voltage, to force these non-spontaneous reactions to happen. This energy input overcomes the chemical bonds holding the compound together.
Key Components
Every electrolysis cell has three primary components:
- Anode: The positive electrode. At the anode, negatively charged ions (anions) lose electrons in a process called oxidation.
- Cathode: The negative electrode. At the cathode, positively charged ions (cations) gain electrons in a process called reduction.
- Electrolyte: A substance (often a solution) containing free-moving ions. The electrolyte conducts electricity and provides the raw material for the reaction.
The type of electrodes and the specific electrolyte used are carefully chosen because they directly determine what products will be formed.
Major Industrial Applications
The ability to precisely control chemical transformations with electricity makes electrolysis a cornerstone of modern industry.
Hydrogen Production from Water
Perhaps the most discussed application today is water electrolysis. By passing a current through water (containing a suitable electrolyte), the cell splits H₂O molecules into their components.
Oxygen gas forms at the anode, while pure hydrogen gas forms at the cathode. When the electricity used is from renewable sources, the resulting product is called "green hydrogen," a key component in a future clean energy economy.
Metal Refining and Plating
Electrolysis is critical for producing and purifying metals. In electroplating, an object is placed as the cathode in a cell containing ions of the desired plating metal (like chromium or nickel). The metal ions are reduced onto the object, forming a thin, durable coating.
Similarly, electrorefining is used to produce ultra-pure metals. An impure slab of copper, for example, is used as the anode. When current is applied, the copper atoms dissolve into the electrolyte, travel to the cathode, and redeposit as nearly 100% pure copper, leaving impurities behind.
The Chlor-Alkali Process
This massive industrial process is one of the most significant uses of electrolysis. A strong solution of sodium chloride (brine) is electrolyzed.
The result is the production of three highly valuable commodity chemicals from simple saltwater: chlorine gas, hydrogen gas, and sodium hydroxide (caustic soda). These are foundational ingredients for manufacturing plastics, paper, soaps, and thousands of other products.
Understanding the Trade-offs
While powerful, electrolysis is not a universal solution. Its application involves significant considerations.
High Energy Consumption
The primary drawback of electrolysis is its high energy requirement. Forcing a stable compound to break apart requires a substantial amount of electrical power, which can make the process expensive. The economic viability of an electrolytic process is often directly tied to the cost of electricity.
Material Sensitivity and Corrosion
The electrodes themselves are part of an active chemical environment. They must be able to withstand corrosive conditions and high temperatures without degrading or reacting in unwanted ways. Choosing the right electrode material is crucial for efficiency and longevity.
Managing Byproducts
Electrolysis can produce hazardous or difficult-to-handle byproducts. For instance, in the chlor-alkali process, the chlorine gas produced is toxic and must be managed with extreme care. The overall environmental impact depends heavily on both the source of electricity and the safe handling of all outputs.
Making the Right Choice for Your Goal
The design and operation of an electrolysis cell are tailored specifically to its intended purpose.
- If your primary focus is producing high-purity metals: Your process will center on electrorefining or electrowinning, where precise voltage control and electrolyte purity are paramount to ensure product quality.
- If your primary focus is generating commodity chemicals: You will likely use a model like the chlor-alkali process, which is optimized for continuous, large-scale production from an inexpensive feedstock like brine.
- If your primary focus is creating clean energy carriers: Your efforts will involve water electrolysis, where cell efficiency and integration with low-cost, renewable energy sources are the most critical factors for success.
By applying targeted electrical energy, an electrolysis cell transforms basic inputs into high-value outputs, making it a foundational tool of modern chemistry and industry.
Summary Table:
| Application | Key Products | Primary Use Case |
|---|---|---|
| Water Electrolysis | Hydrogen & Oxygen Gas | Clean Energy (Green Hydrogen) |
| Metal Refining/Plating | Ultra-Pure Metals, Protective Coatings | Manufacturing, Electronics |
| Chlor-Alkali Process | Chlorine, Hydrogen, Sodium Hydroxide | Plastics, Paper, Soap Production |
Ready to integrate electrolysis technology into your lab or industrial process? KINTEK specializes in high-quality lab equipment and consumables, providing the reliable tools you need for efficient and precise chemical transformations. Whether you're developing clean energy solutions or refining materials, our expertise supports your goals. Contact our team today to discuss how we can enhance your operations.
Visual Guide
Related Products
- Electrolytic Electrochemical Cell with Five-Port
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- H Type Electrolytic Cell Triple Electrochemical Cell
- Electrolytic Electrochemical Cell for Coating Evaluation
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
People Also Ask
- What general precaution should be taken when handling the electrolytic cell? Ensure Safe and Accurate Lab Results
- What material is the five-port water bath electrolytic cell made of? High Borosilicate Glass & PTFE Explained
- How can leaks be prevented when using a five-port water bath electrolytic cell? Ensure a Reliable and Safe Electrochemical Setup
- How should the five-port water bath electrolytic cell be cleaned for maintenance? A Step-by-Step Guide to Reliable Results
- How can contamination be avoided during experiments with the five-port water bath electrolytic cell? Master the 3-Pillar Protocol



















