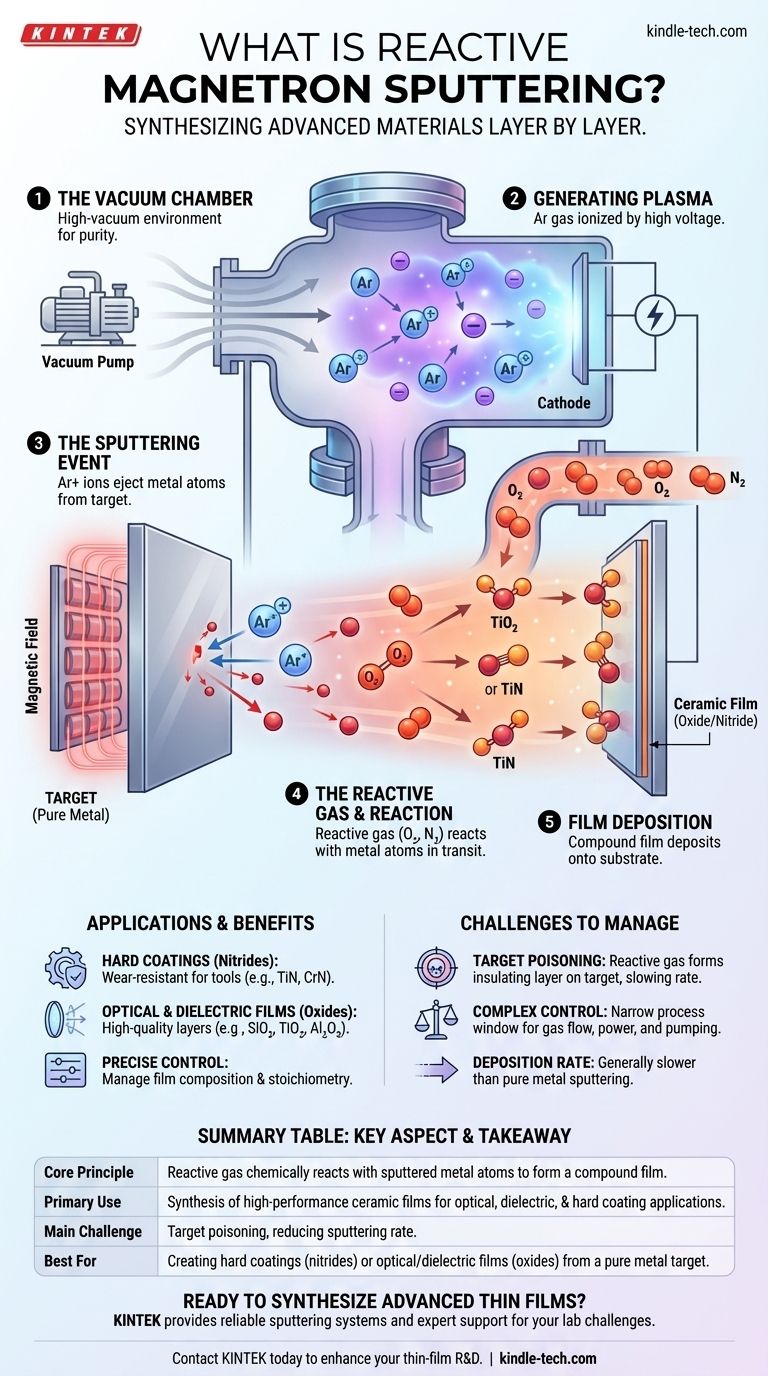
In essence, reactive magnetron sputtering is a highly versatile thin-film deposition technique used to create compound materials. It builds upon the standard magnetron sputtering process by intentionally introducing a reactive gas, such as oxygen or nitrogen, into the vacuum chamber. This gas chemically reacts with the sputtered metal atoms, forming a new compound material—like an oxide or nitride—that deposits onto the substrate.
The core principle is simple yet powerful: instead of just depositing a pure metal, you use a reactive gas to synthesize a new compound material directly onto your substrate during the deposition process itself. This transforms a physical deposition method into a tool for controlled chemical creation.

The Foundation: How Standard Magnetron Sputtering Works
To understand the reactive process, we must first understand its foundation. Standard magnetron sputtering is a Physical Vapor Deposition (PVD) method that involves several key steps.
The Vacuum Environment
The entire process occurs within a high-vacuum chamber. Removing air and other contaminants is critical to ensure the purity of the final film and allow sputtered atoms to travel freely to the substrate.
Generating the Plasma
A low-pressure inert gas, almost always argon (Ar), is introduced into the chamber. A high voltage is then applied, which strips electrons from the argon atoms, creating a glowing, ionized gas known as a plasma. This plasma consists of positively charged argon ions and free electrons.
The Sputtering Event
The material to be deposited, known as the target, is given a strong negative charge. This attracts the positively charged argon ions from the plasma, which accelerate towards the target at high speed. When these ions collide with the target, their momentum is forceful enough to knock out, or "sputter," individual atoms from the target's surface.
The Role of the Magnetic Field
This is the "magnetron" part of the name. A powerful magnetic field is configured behind the target to trap the light, negatively charged electrons from the plasma. This confinement dramatically increases the density of the plasma near the target, which significantly boosts the rate of ion creation and, therefore, the sputtering efficiency. This allows for faster deposition rates at lower gas pressures.
The Critical Difference: Introducing the Reactive Gas
Reactive sputtering takes the foundational process and adds one crucial ingredient that changes the outcome entirely.
What is a Reactive Gas?
While the inert argon gas creates the plasma, a second, chemically reactive gas is carefully bled into the chamber. The most common reactive gases are oxygen (O₂) for creating oxide films and nitrogen (N₂) for creating nitride films.
How the Reaction Occurs
As atoms are sputtered from the pure metal target (e.g., Titanium), they travel through the chamber. During this transit, they collide and react with the molecules of the reactive gas. This chemical reaction forms a new compound (e.g., Titanium + Oxygen → Titanium Dioxide, TiO₂). This newly formed compound then continues to the substrate and deposits as a thin film.
Why Use This Method?
This technique allows for the creation of high-performance ceramic films, such as dielectrics, hard coatings, or optical layers, using a standard, easy-to-fabricate pure metal target. It provides precise control over the film's chemical composition, or stoichiometry, by carefully managing the flow rate of the reactive gas.
Understanding the Trade-offs and Challenges
While powerful, reactive sputtering introduces complexities that require careful management.
The "Poisoning" Effect
The most significant challenge is target poisoning. This occurs when the reactive gas reacts not only with the sputtered atoms but also with the surface of the target itself. This can form an insulating layer on the target, which dramatically reduces the sputtering rate and can make the process unstable.
Process Control Complexity
The process window for stable deposition can be very narrow. It requires sophisticated feedback systems to precisely balance the reactive gas flow, the pumping speed, and the power applied to the magnetron. A slight imbalance can either lead to a poorly-reacted film or a completely poisoned target.
Deposition Rate Reduction
Generally, the deposition rates for reactive sputtering are lower than for sputtering a pure metal. The reaction on the target surface and the overall process dynamics often slow down the rate at which material can be deposited onto the substrate.
Making the Right Choice for Your Goal
Reactive magnetron sputtering is not a universal solution; it is a specialized tool for creating specific types of advanced materials.
- If your primary focus is creating hard, wear-resistant coatings: Use reactive sputtering with nitrogen to deposit nitrides like Titanium Nitride (TiN) or Chromium Nitride (CrN).
- If your primary focus is producing high-quality optical or dielectric films: Use reactive sputtering with oxygen to deposit oxides like Silicon Dioxide (SiO₂), Titanium Dioxide (TiO₂), or Aluminum Oxide (Al₂O₃).
- If your primary focus is depositing a pure metallic film at the highest possible speed: Do not use reactive sputtering; the standard, non-reactive process with only argon gas is the correct choice.
Ultimately, reactive magnetron sputtering transforms a simple physical deposition process into a versatile tool for chemical synthesis, enabling the creation of advanced materials layer by layer.
Summary Table:
| Aspect | Key Takeaway |
|---|---|
| Core Principle | A reactive gas (e.g., O₂, N₂) chemically reacts with sputtered metal atoms to form a compound film (e.g., oxide, nitride). |
| Primary Use | Synthesis of high-performance ceramic films for optical, dielectric, and hard coating applications. |
| Main Challenge | Target poisoning, where the reactive gas forms an insulating layer on the target, reducing sputtering rate. |
| Best For | Creating hard coatings (nitrides) or optical/dielectric films (oxides) from a pure metal target. |
Ready to Synthesize Advanced Thin Films in Your Lab?
Reactive magnetron sputtering is a powerful technique, but its success depends on precise control and the right equipment. KINTEK specializes in lab equipment and consumables, providing the reliable sputtering systems and expert support you need to master this process.
We help our laboratory customers overcome challenges like target poisoning and achieve stable, high-quality deposition of oxides, nitrides, and other compound films.
Contact KINTEK today to discuss your specific application and discover how our solutions can enhance your thin-film research and development.
Visual Guide
Related Products
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
People Also Ask
- What is plasma activated chemical vapour deposition method? A Low-Temperature Solution for Advanced Coatings
- What are the advantages of plasma enhanced CVD? Enable Low-Temperature, High-Quality Thin Film Deposition
- What are the uses of PECVD? A Guide to Low-Temperature Thin-Film Deposition
- What is the plasma CVD process? Achieve Low-Temperature Thin Film Deposition
- What are the benefits of PECVD? Achieve Superior Low-Temperature Thin Film Deposition



















