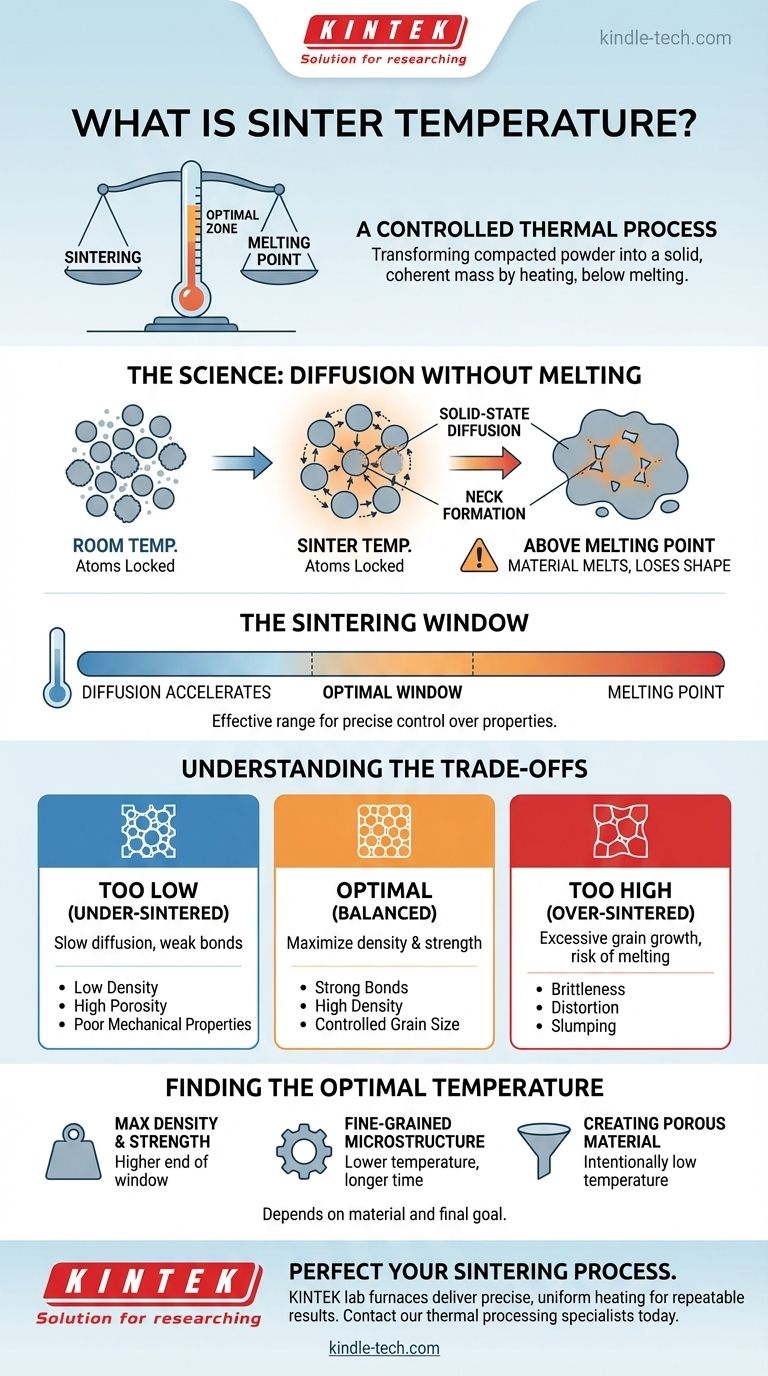
In essence, sinter temperature is the specific, controlled temperature used to transform a compacted powder into a solid, coherent mass. This process, known as sintering, works by heating the material to a point high enough to cause atoms to bond between particles but low enough to avoid melting the entire object.
The core challenge of sintering is a balancing act. The temperature must be hot enough to energize atoms to diffuse and weld particles together, yet it must remain strictly below the material's melting point to maintain the part's shape and integrity.

The Science Behind Sintering: Diffusion Without Melting
Sintering is a thermal process that gives powdered materials strength and density. The selected temperature is the single most critical variable controlling the outcome.
Activating Atomic Diffusion
At room temperature, the atoms in a compacted powder are locked in place. As the material is heated to its sinter temperature, the atoms gain enough thermal energy to move.
This movement, called solid-state diffusion, allows atoms to migrate across the boundaries of contacting particles. They form small bridges, or "necks," that fuse the individual particles together, turning a loose compact into a strong, unified part.
Why Staying Below the Melting Point is Critical
If the temperature exceeds the material's melting point, the process is no longer sintering; it's melting. The material will liquefy, lose its shape, and collapse.
The goal of sintering is to achieve densification and strength while the material is still in a solid state. This preserves the intended geometry of the component, which is impossible if widespread melting occurs.
The Concept of a "Sintering Window"
For any given material, there isn't one single perfect temperature but rather a "sintering window." This is the effective temperature range between the point where diffusion begins to accelerate and the material's melting point.
Operating within this window allows for precise control over the final properties of the material.
Understanding the Trade-offs in Temperature Selection
Choosing a temperature within the sintering window involves critical trade-offs that directly impact the final product's quality and performance.
The Risk of Insufficient Temperature
If the temperature is too low, atomic diffusion will be slow and incomplete. This results in weak bonds between particles.
The final part will suffer from low density, high porosity, and poor mechanical properties. This is known as an "under-sintered" state.
The Danger of Excessive Temperature
If the temperature is too high, even if it's below the melting point, negative effects can occur. The primary risk is excessive grain growth, where smaller crystal grains merge into larger ones. This can often make the material more brittle.
In extreme cases, temperatures approaching the melting point can cause the part to slump, distort, or undergo localized melting, ruining its dimensional accuracy. This is an "over-sintered" state.
Balancing Density and Grain Size
There is a constant tension between achieving high density and maintaining a fine grain structure. Higher temperatures generally increase the rate of densification but also accelerate grain growth.
The ideal sinter temperature is one that maximizes density while keeping the grain size within the desired specification for the material's application.
Finding the Optimal Sinter Temperature
The right temperature is not a universal constant but depends entirely on your material and your final goal.
- If your primary focus is maximum density and strength: You will likely operate toward the higher end of the material's sintering window, carefully managing time to prevent excessive grain growth.
- If your primary focus is preserving a fine-grained microstructure for toughness or hardness: You may use a lower temperature for a longer duration or employ advanced sintering methods to control grain growth.
- If your primary focus is creating a porous material (e.g., for a filter): You will intentionally use a lower temperature to form strong inter-particle bonds without fully closing the gaps between them.
Ultimately, mastering the sinter temperature is the key to precisely engineering the final microstructure and performance of your component.
Summary Table:
| Sinter Temperature Effect | Outcome |
|---|---|
| Too Low | Weak bonds, low density, high porosity (under-sintered) |
| Optimal | Strong bonds, high density, controlled grain size |
| Too High | Excessive grain growth, distortion, brittleness (over-sintered) |
Ready to Perfect Your Sintering Process?
Achieving the precise sinter temperature is critical for the density, strength, and microstructure of your components. The experts at KINTEK understand the delicate balance required for successful sintering.
We provide high-quality lab furnaces and consumables that deliver the precise, uniform heating essential for repeatable results. Whether you are working with metals, ceramics, or advanced alloys, our equipment is designed to help you master your thermal processing.
Let KINTEK be your partner in precision.
Contact our thermal processing specialists today to discuss how our solutions can optimize your sintering outcomes and improve your product performance.
Visual Guide
Related Products
- Dental Porcelain Zirconia Sintering Ceramic Furnace Chairside with Transformer
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
- Vacuum Heat Treat and Sintering Furnace with 9MPa Air Pressure
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Vacuum Dental Porcelain Sintering Furnace
People Also Ask
- What are the white spots on zirconia after sintering? A Guide to Diagnosing and Preventing Defects
- What is the price of zirconia sintering furnace? Invest in Precision, Not Just a Price Tag
- What is the sintering temperature of zirconium? A Guide to the 1400°C-1600°C Range for Dental Labs
- What is the effect of zirconia sintering temperature? Master the Key to Strength and Stability
- Can you change the color of zirconia crowns? Understanding the Permanent Nature of Zirconia



















