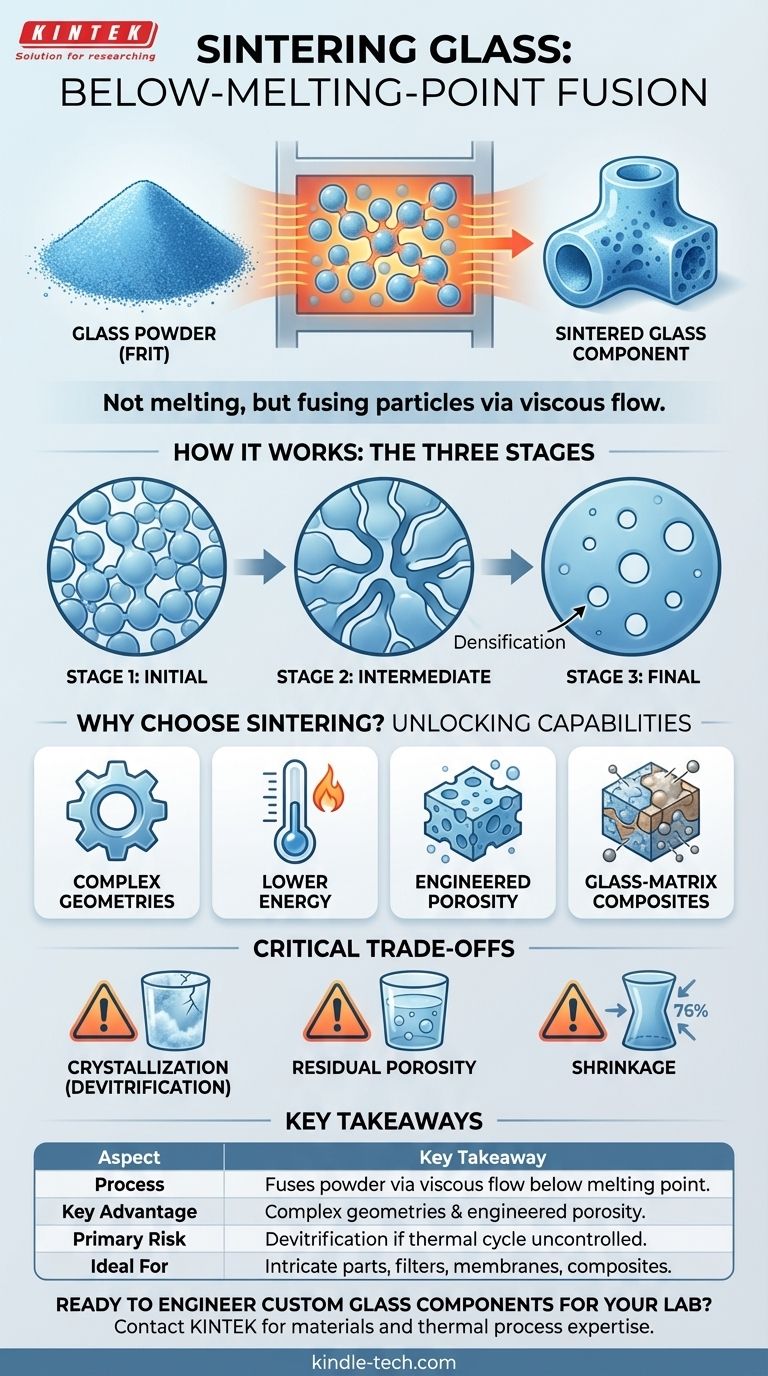
In essence, sintering glass is a thermal process used to consolidate glass powder (known as "frit") into a solid mass at a temperature below its melting point. Instead of fully melting the material into a liquid, sintering causes the individual glass particles to fuse together at their contact points, gradually reducing the porous space between them and creating a dense, solid object.
The core concept to grasp is that sintering is not melting. It is a method of forming glass components by making the material soft enough to stick together, enabling the creation of complex shapes and engineered materials that are impossible to achieve with traditional glass-melting techniques.

How Does Glass Sintering Actually Work?
The process is driven by fundamental physics and controlled by precise thermal management. It transforms a loose powder into a coherent solid.
The Starting Point: Glass Powder (Frit)
The process begins with glass that has been crushed and milled into a fine powder, often called glass frit. The size and shape distribution of these powder particles are critical parameters that influence the final properties of the sintered part.
The Driving Force: Reducing Surface Energy
A large volume of fine powder has an enormous amount of surface area, which corresponds to high surface energy. Nature inherently seeks the lowest energy state. By bonding together and reducing the space between particles, the system drastically reduces its total surface area, thus lowering its overall energy.
The Key Mechanism: Viscous Flow
As the glass powder is heated, it doesn't liquefy. Instead, its viscosity (resistance to flow) decreases significantly. The glass becomes soft and deformable, allowing it to slowly flow and form "necks" or bridges between adjacent particles.
Think of it less like melting an ice cube into water and more like sticky honey drops slowly merging into a single, larger mass. This viscous flow is the mechanism that closes the pores and densifies the material.
The Stages of Densification
The process generally occurs in three overlapping stages:
- Initial Stage: Necks form and grow between particles, but the pores are still largely interconnected.
- Intermediate Stage: The structure densifies rapidly. Shrinkage is significant as the pores form a continuous, channel-like network.
- Final Stage: The pores become isolated and spherical. The final traces of porosity are slowly eliminated to achieve full density, which is often the most challenging stage.
Why Choose Sintering Over Traditional Melting?
Sintering unlocks capabilities that are simply not feasible with conventional melt-processing, where glass is melted into a liquid and then cast, blown, or molded.
Creating Complex Geometries
Sintering is excellent for producing near-net-shape parts with intricate designs. The glass powder can be molded or pressed into a complex shape (a "green body") first and then heated, a process that is far more versatile than trying to mold molten glass. This is the foundational principle behind 3D printing of glass.
Working at Lower Temperatures
Because sintering occurs below the melting point, it requires less energy and less extreme furnace conditions than full melting. This can also be crucial when working with glasses that have a tendency to degrade or react at higher temperatures.
Engineering Porosity
By carefully controlling the sintering time and temperature, the process can be halted before full densification. This allows for the creation of porous glass structures with a defined pore size, which are invaluable for applications like scientific filters, membranes, and biomedical scaffolds.
Making Glass-Matrix Composites
Sintering allows for the mixing of glass powder with other materials, such as ceramics or metals. This makes it possible to create composite materials that combine the properties of both, which would be impossible if the glass had to be fully melted.
Understanding the Critical Trade-offs
While powerful, sintering is a complex process with significant challenges that must be managed to achieve a successful outcome.
The Challenge of Crystallization (Devitrification)
This is the primary risk. Glass is an amorphous (non-crystalline) material. If held for too long in the sintering temperature range, it can begin to crystallize, a process known as devitrification. This unwanted crystallization makes the glass opaque and brittle, destroying its desired properties. Success depends on working within a precise thermal window.
The Problem of Residual Porosity
Achieving 100% density and eliminating every last pore is extremely difficult. Residual porosity can negatively impact the mechanical strength and, most importantly, the optical clarity of the glass. Trapped gas within the pores is a common culprit.
Managing Shrinkage
As the pores are eliminated, the entire component shrinks. This shrinkage can be substantial (often 15-20% by volume) and must be accurately predicted and accounted for in the initial design of the mold or green body.
How to Apply This to Your Project
Choosing the right glass forming technique depends entirely on the requirements of your final product.
- If your primary focus is intricate geometry or engineered porosity: Sintering is the superior, and often the only, viable manufacturing method.
- If your primary focus is maximum optical clarity and mechanical strength: Traditional melt-processing is generally the more reliable and straightforward path.
- If your primary focus is creating composite materials with a glass matrix: Sintering provides a low-temperature route to combine materials that could not survive a full melt.
Understanding the principles of sintering allows you to move beyond the limits of conventional glasswork and engineer materials with truly novel forms and functions.
Summary Table:
| Aspect | Key Takeaway |
|---|---|
| Process | Fuses glass powder (frit) below its melting point via viscous flow. |
| Key Advantage | Enables creation of complex geometries and engineered porous structures. |
| Primary Risk | Devitrification (unwanted crystallization) if temperature/time not controlled. |
| Ideal For | Intricate parts, filters, membranes, composites, and near-net-shape manufacturing. |
Ready to engineer custom glass components for your lab?
Sintering glass unlocks possibilities for specialized lab equipment, from intricate reactor parts to porous filters and unique composite materials. At KINTEK, we specialize in providing the high-quality lab equipment and consumables you need to succeed.
Our experts can help you select the right materials and understand the thermal processes for your specific application. Let's discuss how we can support your R&D or production goals.
Contact our team today to explore the potential of sintered glass in your laboratory.
Visual Guide
Related Products
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
- 1800℃ Muffle Oven Furnace for Laboratory
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- Why is a quartz tube furnace utilized in the thermal oxidation of MnCr2O4 coatings? Unlock Precise Selective Oxidation
- What precautions should be taken when using a tube furnace? Ensure Safe, Effective High-Temperature Processing
- What is the basic construction and temperature control mechanism of a laboratory tube furnace? Master Precision Heating for Your Lab
- What is a tubular furnace used for? Precision Heating for Material Synthesis & Analysis
- What is the technical value of using a quartz tube reaction chamber for static corrosion testing? Achieve Precision.



















