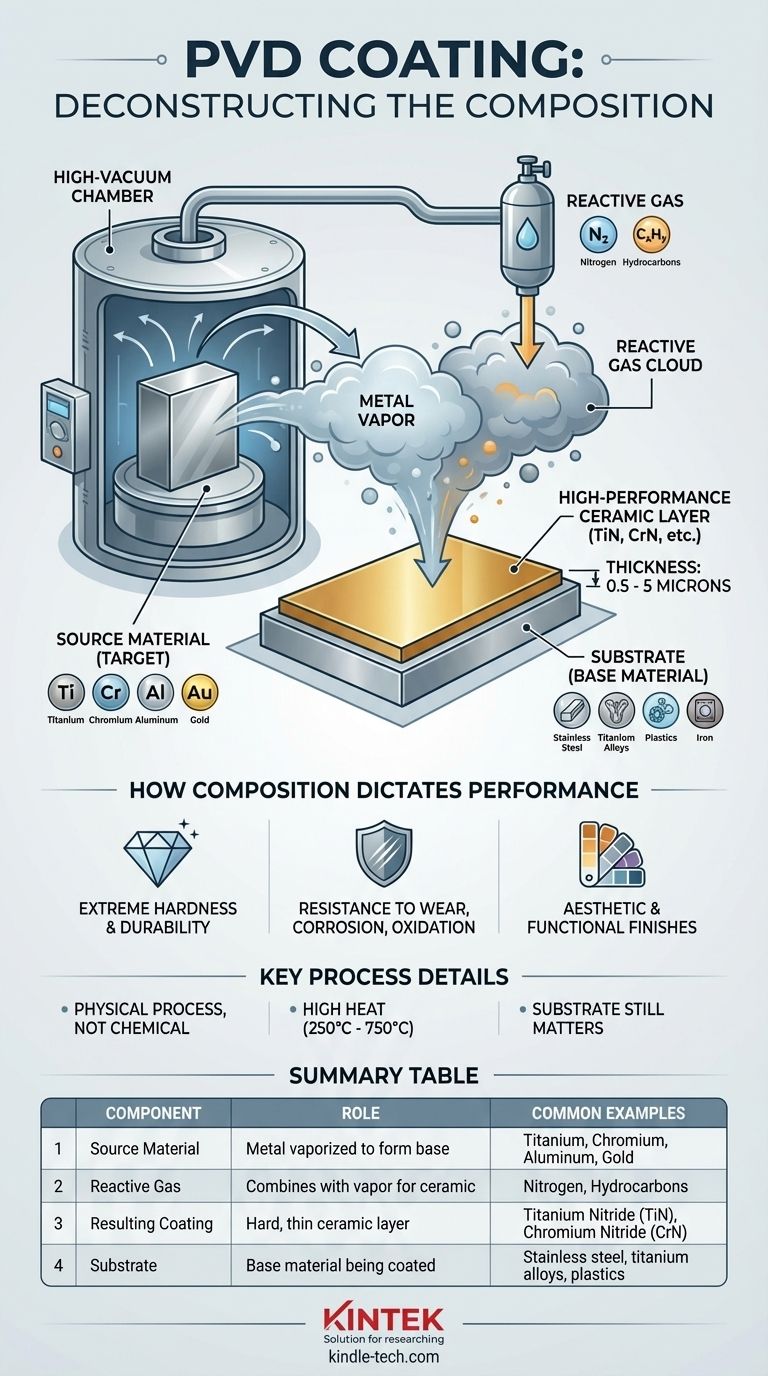
At its core, a PVD coating is not a single substance. It is a composite material formed by combining a source metal—most commonly titanium, chromium, or aluminum—with a reactive gas like nitrogen or a hydrocarbon. This process creates an extremely hard and thin ceramic layer, such as Titanium Nitride (TiN), which bonds directly to the surface of the base material on a molecular level.
The key takeaway is that PVD coating composition is a strategic formula, not a simple paint. It involves vaporizing a metal in a vacuum and reacting it with a specific gas to create a new, high-performance ceramic compound on the surface of an object.

Deconstructing PVD: Source, Gas, and Substrate
To truly understand the composition, you must look at the three key elements involved in the Physical Vapor Deposition (PVD) process.
The Source Material (The "Target")
The foundation of the coating is a solid source material, known as the target. This material is what gets vaporized inside the vacuum chamber.
Common source metals include titanium, chromium, tungsten, and aluminum. For decorative or specific functional purposes, precious metals like gold or alloys like brass can also be used as the target.
The Reactive Gas
A pure vaporized metal would offer limited benefits. The transformative step involves introducing a carefully controlled reactive gas into the vacuum chamber.
This gas combines with the metal vapor as it deposits onto the part. The most common gas is nitrogen, which creates highly durable nitride coatings. Hydrocarbon-based gases can also be used to form carbonitride coatings.
The Substrate (The Base Material)
The substrate is the object being coated. The PVD process is highly versatile and compatible with a wide range of materials.
Substrates can include stainless steel, titanium alloys, plastics, iron, and gold. The final properties of the finished product are a combination of both the coating and the underlying substrate material.
How Composition Dictates Performance
The specific combination of source metal and reactive gas is chosen to achieve a desired outcome. This molecular-level engineering is what gives PVD coatings their remarkable properties.
Creating a High-Performance Ceramic Layer
The essence of the PVD process is turning a relatively soft metal into an exceptionally hard ceramic. For example, titanium metal reacts with nitrogen gas to form Titanium Nitride (TiN), the most common PVD coating.
This new compound is a ceramic, which is fundamentally harder and more resistant to wear than the original metal. The resulting layer is extremely thin, typically between 0.5 and 5 microns.
Hardness and Durability
The final nitride or carbonitride composition is what provides the exceptional hardness and durability. This thin film dramatically increases resistance to scratches, corrosion, and oxidation.
For example, a TiN coating applied to a titanium alloy can significantly increase its fatigue limit and endurance, making the part last much longer under stress.
Aesthetic and Functional Finishes
Beyond durability, the composition directly controls the final look. Different source metals, gases, and process parameters can produce a wide spectrum of colors and textures.
This is why PVD is used for everything from durable black tool bits to high-end gold-colored watches and fixtures.
Understanding the Trade-offs and Process
While the results are impressive, it's important to recognize that PVD is a sophisticated industrial process with specific requirements.
It's a Physical, Not Chemical, Process
The name "Physical Vapor Deposition" highlights a key distinction. The source material is a physical solid that is vaporized, not a chemical precursor gas as in Chemical Vapor Deposition (CVD). This makes the PVD process more environmentally friendly.
The Role of Heat and Vacuum
The PVD process must be performed in a high-vacuum chamber at elevated temperatures, often ranging from 250°C to 750°C.
This high-heat requirement means that the substrate material must be able to withstand the process temperatures without deforming or degrading.
The Substrate Still Matters
A PVD coating is an enhancement, not a replacement for the base material. The overall strength, flexibility, and performance of a part are still primarily determined by the underlying substrate. The coating provides surface protection, but it cannot fix a weak foundation.
Making the Right Choice for Your Goal
The "best" PVD composition is entirely dependent on the intended application.
- If your primary focus is extreme hardness and wear resistance: A composition like Titanium Nitride (TiN) or Chromium Nitride (CrN) is a standard, highly effective choice for tools and industrial parts.
- If your primary focus is a specific decorative finish: The composition will be chosen based on the desired color, using source metals like titanium, zirconium, or even real gold to achieve the final aesthetic.
- If your primary focus is corrosion resistance in a demanding environment: A multi-layered or specialized composition will be engineered to provide a durable, non-reactive barrier on the specific substrate.
Ultimately, the composition of a PVD coating is a precise engineering choice designed to bond a high-performance ceramic layer to a substrate for superior durability and function.
Summary Table:
| Component | Role in PVD Coating | Common Examples |
|---|---|---|
| Source Material (Target) | The metal vaporized to form the coating base. | Titanium, Chromium, Aluminum, Gold |
| Reactive Gas | Combines with metal vapor to create a ceramic compound. | Nitrogen (for nitrides), Hydrocarbons (for carbonitrides) |
| Resulting Coating | The final, hard, thin ceramic layer bonded to the substrate. | Titanium Nitride (TiN), Chromium Nitride (CrN) |
| Substrate | The base material being coated. | Stainless steel, titanium alloys, plastics |
Ready to engineer the perfect surface for your application?
The specific composition of a PVD coating is key to achieving the exact hardness, durability, color, and corrosion resistance you need. At KINTEK, we specialize in providing the lab equipment and consumables necessary for developing and applying advanced PVD coatings.
Whether you are researching new coating formulas or scaling up production, our expertise supports your laboratory's success. Contact our experts today to discuss how we can help you achieve superior surface performance.
Visual Guide
Related Products
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
- VHP Sterilization Equipment Hydrogen Peroxide H2O2 Space Sterilizer
- Molybdenum Tungsten Tantalum Special Shape Evaporation Boat
People Also Ask
- What are the advantages of PECVD? Enable Low-Temperature, High-Quality Thin-Film Deposition
- What is plasma activated chemical vapour deposition method? A Low-Temperature Solution for Advanced Coatings
- How are PECVD and CVD different? A Guide to Choosing the Right Thin-Film Deposition Process
- What are the applications of PECVD? Essential for Semiconductors, MEMS, and Solar Cells
- How does RF power create plasma? Achieve Stable, High-Density Plasma for Your Applications



















