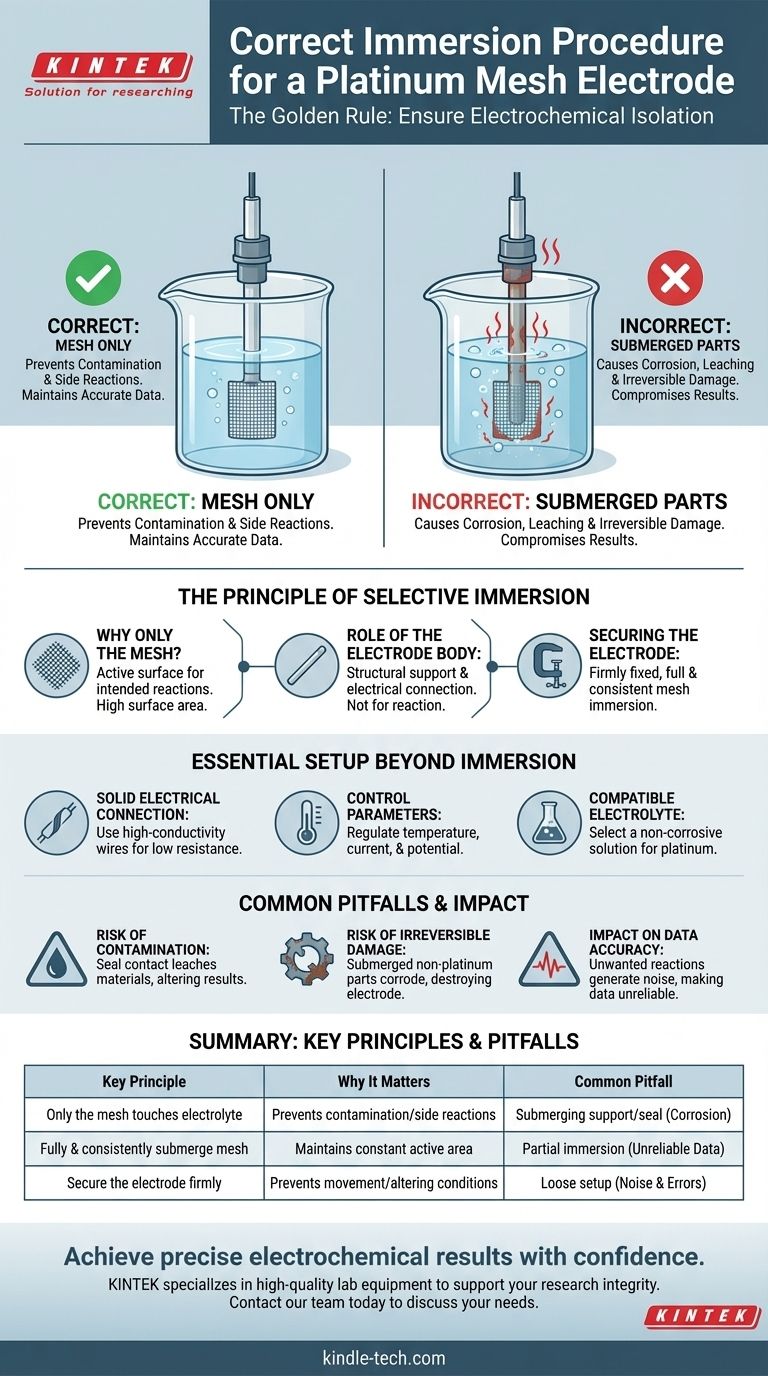
The single most important rule for using a platinum mesh electrode is that only the platinum mesh itself should ever touch the electrolyte solution. Submerging any other part of the electrode, such as the connecting rod or the seal, is strictly forbidden as it will compromise your experiment and can permanently damage the equipment.
The core principle is not just about immersion depth; it's about electrochemical isolation. Your goal is to ensure that only the pure, inert platinum surface participates in the reaction, preventing contamination and electrical interference from the electrode's structural components.

The Principle of Selective Immersion
The design of a platinum mesh electrode is intentional. Each part has a specific function, and understanding these roles is key to using the tool correctly.
Why Only the Mesh?
The platinum mesh is the active surface of the electrode. This is where the intended electrochemical reactions (oxidation or reduction) occur. Its high surface area and catalytic properties are the reasons you selected it.
If other parts of the electrode contact the solution, they can introduce unwanted side reactions, leach impurities into your electrolyte, or corrode.
The Role of the Electrode Body
The rod or stem holding the platinum mesh is typically made of an inert material like glass or a conductive metal encased in PTFE (Teflon). Its purpose is purely for structural support and electrical connection, not for participating in the experiment.
These materials are not designed to withstand the electrochemical conditions within your cell and can quickly degrade if submerged.
Securing the Electrode Correctly
You must fix the electrode securely within your electrolytic cell using a holder or clamp. The positioning should ensure the entire platinum mesh is fully and consistently submerged in the electrolyte for the duration of the experiment.
Any movement or partial immersion will change the active surface area and make your results unreliable and difficult to repeat.
Essential Setup Beyond Immersion
Proper immersion is the first step. To ensure a successful experiment, you must also control the surrounding electrical and environmental conditions.
Establishing a Solid Electrical Connection
Use high-conductivity wires and clips to connect the electrode's terminal to your power source, such as a potentiostat or electrochemical workstation. A firm, low-resistance connection is critical for accurate potential control and current measurement.
Controlling Experimental Parameters
Your experimental protocol dictates the conditions. You must control parameters like temperature, current density, and potential according to your requirements. Most platinum electrodes are designed for use at room temperature unless specified otherwise.
Choosing a Compatible Electrolyte
Select an electrolyte that is appropriate for your reaction and, crucially, does not corrode platinum. While platinum is highly inert, aggressive solutions or certain conditions can still cause degradation.
Common Pitfalls and How to Avoid Them
Mistakes during setup are the primary source of inaccurate data and damaged equipment. Understanding these risks is essential for prevention.
The Risk of Contamination
If the seal between the platinum mesh and the support rod touches the electrolyte, materials can leach from the seal into your solution. This chemical contamination can alter your results in unpredictable ways.
The Risk of Irreversible Damage
Submerging the connection point or the non-platinum rod can cause catastrophic corrosion. The current will attack the less-noble materials, destroying the electrode's internal connection and rendering it useless. This is an expensive and entirely avoidable mistake.
The Impact on Data Accuracy
Unintended electrochemical reactions occurring on the support rod or connectors will generate electrical noise and false current signals. This makes it impossible to trust the data you are collecting from the platinum mesh itself.
Applying This to Your Experiment
Your specific goal will determine which aspect of the setup is most critical.
- If your primary focus is data integrity: Ensure only the platinum mesh is submerged to prevent any possibility of side reactions or signal interference.
- If your primary focus is equipment longevity: Pay strict attention to keeping the electrolyte away from the electrode's seals and connection points to prevent corrosion.
- If your primary focus is result repeatability: Standardize your immersion depth and secure the electrode firmly to maintain a constant active surface area in every trial.
Ultimately, precise and careful setup is the foundation of credible electrochemical research.
Summary Table:
| Key Principle | Why It Matters | Common Pitfall |
|---|---|---|
| Only the platinum mesh touches the electrolyte | Prevents contamination and unwanted side reactions. | Submerging the support rod or seal, causing corrosion. |
| Fully and consistently submerge the mesh | Maintains a constant active surface area for repeatable results. | Partial immersion, leading to unreliable data. |
| Secure the electrode firmly | Prevents movement that alters the electrochemical conditions. | Loose setup, introducing noise and errors. |
Achieve precise and contamination-free electrochemical results with confidence.
Proper electrode handling is fundamental to successful experiments. KINTEK specializes in high-quality lab equipment and consumables, including reliable electrochemical cells and accessories, to support your research integrity. Our experts can help you select the right tools and provide guidance on best practices.
Ensure your next experiment is a success. Contact our team today to discuss your specific laboratory needs and how we can support your work.
Visual Guide
Related Products
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Platinum Auxiliary Electrode for Laboratory Use
- Ultra-Vacuum Electrode Feedthrough Connector Flange Power Electrode Lead for High-Precision Applications
- Custom PTFE Teflon Parts Manufacturer for PTFE Mesh F4 Sieve
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
People Also Ask
- How should a platinum sheet electrode be pretreated before use? Ensure Accurate Electrochemical Measurements
- What are the performance characteristics of platinum sheet electrodes? Unlock Superior Electrochemical Performance
- What precautions should be taken when using a platinum sheet electrode? Ensure Accurate & Reproducible Electrochemical Data
- What are the available specifications for platinum sheet electrodes? Find the Perfect Fit for Your Electrochemical Needs
- What is the proper post-treatment procedure for a platinum sheet electrode? Ensure Long-Term Accuracy & Protect Your Investment



















