In thermal analysis, the furnace atmosphere is not a passive background condition; it is an active reactant. The type of gas surrounding a sample directly dictates which chemical reactions can occur during heating, fundamentally altering the material's decomposition pathway, thermal stability, and the resulting data from instruments like TGA or DSC. Choosing the wrong atmosphere is one of the most common sources of erroneous and non-reproducible results.
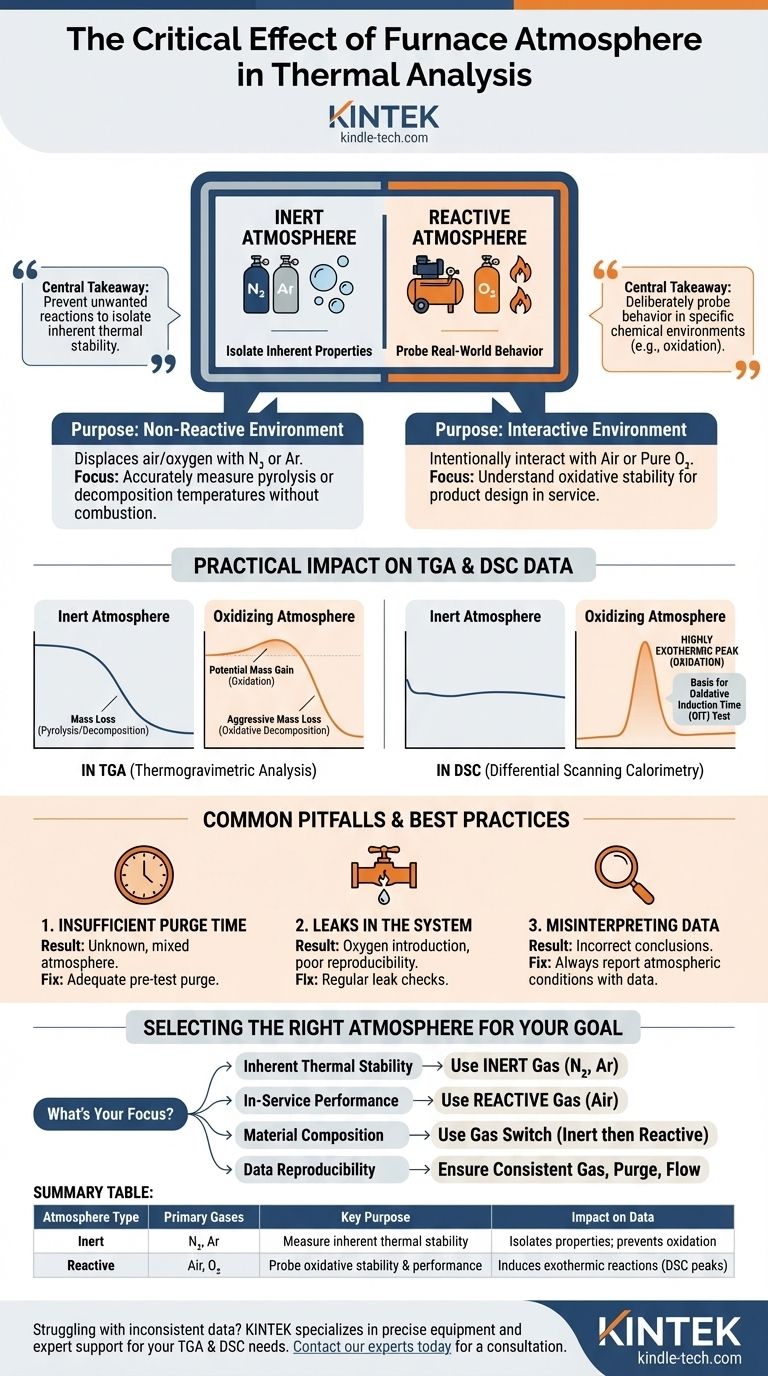
The central takeaway is this: An inert atmosphere (like Nitrogen) is used to isolate a material's inherent thermal properties by preventing unwanted reactions, while a reactive atmosphere (like air or oxygen) is used to deliberately probe the material's behavior in a specific chemical environment, such as its resistance to oxidation.

The Atmosphere's Role: Inert vs. Reactive
The most critical decision in setting up a thermal analysis experiment is the choice between an inert or a reactive gas environment. This choice determines the very nature of the chemical changes you will measure.
The Purpose of an Inert Atmosphere
An inert atmosphere is designed to be non-reactive with the sample. The goal is to create a controlled environment where the only variable causing change is heat.
Gases like Nitrogen (N₂) or Argon (Ar) are used to continuously purge the furnace, displacing any ambient air and oxygen. This prevents oxidative side reactions from occurring.
Under an inert gas, you can accurately measure a material's inherent thermal stability, such as its pyrolysis or decomposition temperature, without the complicating influence of combustion.
The Purpose of a Reactive Atmosphere
A reactive atmosphere is used to intentionally study the interaction between the sample and a specific gas during heating. The most common reactive atmosphere is air or pure oxygen (O₂).
This setup is crucial for understanding a material's oxidative stability. It helps answer questions about how a material will perform in its real-world service environment where oxygen is present.
For example, measuring the temperature at which a plastic begins to break down in air is often more relevant for product design than knowing its decomposition temperature in a vacuum.
Practical Impacts on Thermal Analysis Data
The choice of atmosphere has a direct and often dramatic effect on the data curves produced by different thermal analysis instruments.
In TGA (Thermogravimetric Analysis)
TGA measures changes in mass versus temperature. In an inert atmosphere, you typically observe a simple mass loss as the material pyrolyzes and decomposes into volatile components.
In an oxidizing atmosphere like air, the TGA curve is far more complex. You might first see a slight mass gain as the material reacts with oxygen, followed by a sharp mass loss at a lower temperature than in nitrogen, as oxidative decomposition is often more aggressive.
In DSC (Differential Scanning Calorimetry)
DSC measures heat flow into or out of a sample. Oxidation is a highly exothermic process, meaning it releases a large amount of heat.
A material heated in air will show a large exothermic peak on the DSC curve corresponding to its oxidation. This effect is completely absent when the same material is run in nitrogen. This principle is the basis for the standard Oxidative Induction Time (OIT) test, which measures an antioxidant's effectiveness.
Common Pitfalls and Best Practices
Failing to properly control the furnace atmosphere can lead to data that is misleading and impossible to reproduce.
Insufficient Purge Time
Before starting a run, the furnace must be purged with the desired gas for an adequate amount of time to completely remove all residual air.
Starting the heat program too early results in an experiment run in an unknown, mixed atmosphere, making the data unreliable. An initial, unexpected oxidative event is a classic sign of an insufficient purge.
Leaks in the System
Even a minuscule leak in a gas line or furnace seal can introduce oxygen into an experiment that is supposed to be inert.
This can cause subtle but significant shifts in decomposition temperatures and is a common cause of poor run-to-run reproducibility. Regular leak checks are a critical maintenance procedure.
Misinterpreting the Data
It is essential to always report the atmospheric conditions alongside the data. A decomposition temperature measured in air is a measure of oxidative stability, not inherent thermal stability.
Confusing these two properties is a frequent mistake that leads to incorrect conclusions about a material's fundamental characteristics.
Selecting the Right Atmosphere for Your Goal
To obtain meaningful results, you must align your choice of atmosphere with the specific question you are trying to answer.
- If your primary focus is determining inherent thermal stability: Use a high-purity inert gas like Nitrogen or Argon to isolate decomposition from oxidation.
- If your primary focus is evaluating in-service performance: Use a reactive gas like air to simulate the material's real-world operational environment.
- If your primary focus is studying a material's composition: Use a sequence of inert followed by reactive gas (a "gas switch" experiment) to separate volatiles, carbon black, and inorganic filler content.
- If your primary focus is ensuring data reproducibility: Always use a consistent gas type, a sufficient pre-test purge time, and a precisely controlled flow rate for every experiment.
By treating the furnace atmosphere as a deliberate experimental variable, you gain precise control over your analysis and unlock far more meaningful insights into your material's behavior.
Summary Table:
| Atmosphere Type | Primary Gases | Key Purpose | Impact on Data |
|---|---|---|---|
| Inert | Nitrogen (N₂), Argon (Ar) | Measure inherent thermal stability (pyrolysis, decomposition) | Isolates material properties; prevents oxidation |
| Reactive | Air, Oxygen (O₂) | Probe oxidative stability & real-world performance | Induces exothermic reactions (e.g., oxidation peaks in DSC) |
Are you struggling with inconsistent or misleading thermal analysis data? The furnace atmosphere is a critical variable that can make or break your results. At KINTEK, we specialize in providing the precise lab equipment and expert support needed to master your thermal analysis workflows. Whether you require reliable inert gas purging systems or configurations for reactive atmosphere studies, our solutions are designed to ensure the accuracy and reproducibility of your TGA and DSC data. Let's optimize your process. Contact our experts today for a consultation.
Visual Guide
Related Products
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
People Also Ask
- What is the use of hydrogen furnace? Achieve Superior Purity in High-Temperature Processing
- What is the cheapest inert gas? Argon is the Cost-Effective Choice for True Inertness
- How can we create a reducing environment? Master the Methods for Precise Chemical Control
- What are inert properties? The Key to Predictable Stability and Control in Your Processes
- What are the advantages of using an atmosphere furnace for low-temperature sintering? Optimize Solid-State Electrolytes
- What are the effects of inert gases? Uncovering Their Hidden Risks and Industrial Uses
- What is brazing? A Guide to Strong, Precise Metal Joining for High-Performance Applications
- What is the inert atmosphere for welding? Protect Your Weld Pool from Contamination



















