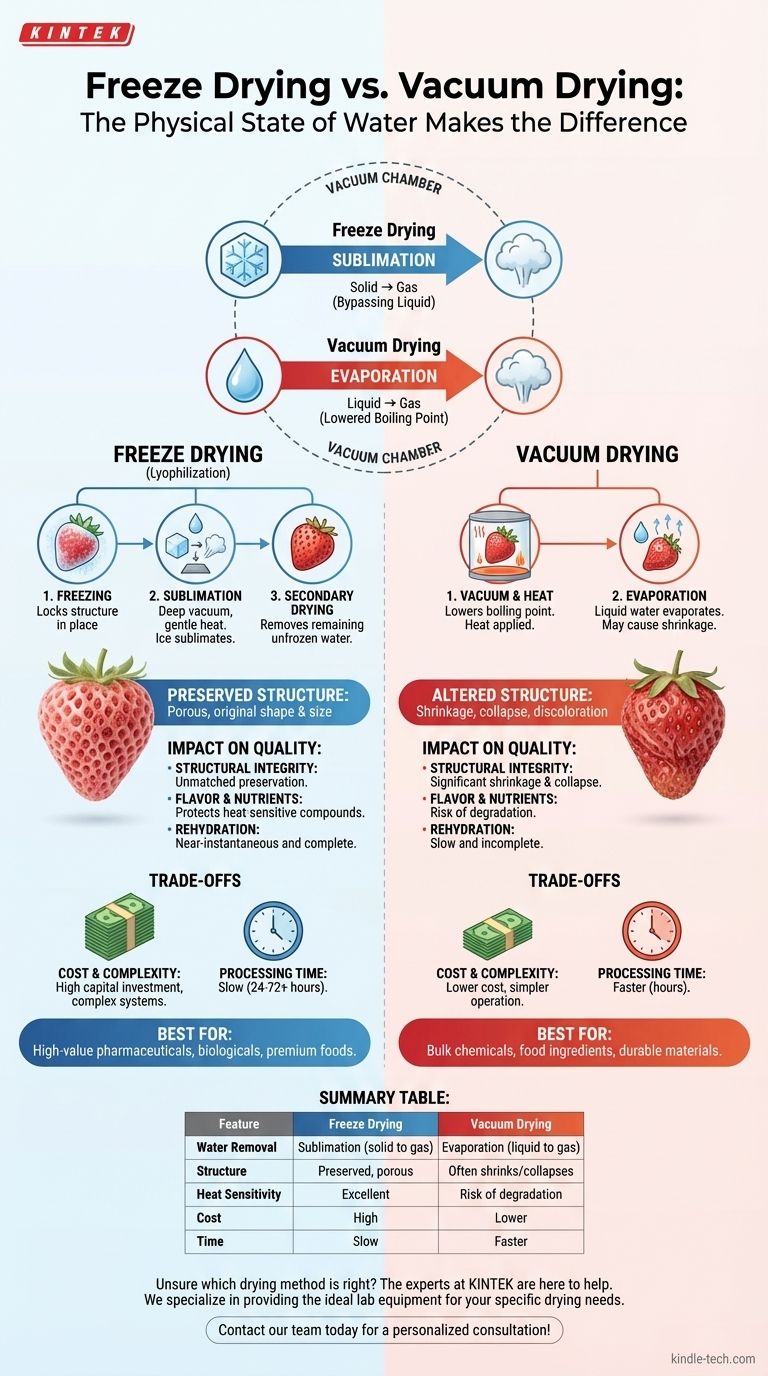
The fundamental difference between freeze drying and vacuum drying lies in the physical state of water as it is removed. Freeze drying works by turning frozen water (ice) directly into a gas through sublimation, bypassing the liquid phase entirely. In contrast, standard vacuum drying uses a vacuum simply to lower water's boiling point, turning liquid water into vapor through evaporation.
While both methods use a vacuum to remove moisture, the choice between them is a critical decision of quality versus efficiency. Freeze drying preserves a product's original structure and delicate properties, whereas vacuum drying offers a faster, more economical process at the potential cost of product integrity.

How Each Process Works: A Tale of Two States
The core distinction is whether water is removed from a solid or a liquid state. This single difference has profound implications for the final product.
Freeze Drying: The Sublimation Principle
Freeze drying, or lyophilization, is a three-stage process designed for maximum preservation. First, the material is frozen solid, locking its structure in place.
Next, a deep vacuum is applied. This pressure is so low that when gentle heat is introduced, the ice crystals do not melt; they sublimate, turning directly from a solid into water vapor.
Finally, a secondary drying phase removes any remaining unfrozen water molecules, resulting in an exceptionally dry and stable product that retains its original shape and size.
Vacuum Drying: The Evaporation Principle
Standard vacuum drying also uses a vacuum, but for a different reason: to lower the boiling point of water. Water that boils at 100°C (212°F) at normal pressure might boil at room temperature under a strong vacuum.
In this process, the material is placed in the chamber, a vacuum is pulled, and heat is applied. The liquid water within the product evaporates into vapor, which is then pumped away.
Because the water is removed as a liquid transitioning to a gas, it can cause the product's structure to shrink or collapse as it vacates the space.
The Impact on Product Quality
The method of water removal directly determines the quality of the final product, from its appearance to its chemical stability.
Structural Integrity and Appearance
Freeze drying is unmatched in preserving a product's physical structure. The ice acts as a scaffold that is removed, leaving a porous, sponge-like matrix that is identical in shape and size to the original.
Vacuum drying, on the other hand, can lead to significant shrinkage and discoloration. The movement of liquid water to the surface can carry solutes with it, and the loss of internal volume often causes the product to collapse on itself.
Preservation of Flavor and Nutrients
The extremely low temperatures used in freeze drying protect heat-sensitive compounds like vitamins, proteins, and flavor molecules. This makes it the gold standard for preserving the sensory and nutritional qualities of food and the efficacy of pharmaceuticals.
The higher temperatures often required for efficient vacuum drying, even with a lowered boiling point, can degrade these delicate compounds.
Rehydration Quality
The porous structure created by freeze drying allows for near-instantaneous and complete rehydration. Water quickly wicks back into the empty spaces left by the sublimated ice.
A vacuum-dried product, with its collapsed and less porous structure, often rehydrates slowly and incompletely.
Understanding the Trade-offs
Choosing a drying method is not just about quality; it's also about balancing operational realities.
Cost and Complexity
Freeze drying is a significantly more complex and expensive process. The equipment required to achieve deep vacuums and precise temperature control is a major capital investment.
Vacuum drying systems are generally simpler, less expensive to acquire, and cheaper to operate.
Processing Time
Freeze drying is exceptionally slow. A typical cycle can last anywhere from 24 to 72 hours or more, depending on the product and thickness.
Vacuum drying is considerably faster, with cycles often completed in a matter of hours.
Making the Right Choice for Your Goal
Your decision must be guided by the specific requirements of your product and your operational constraints.
- If your primary focus is preserving the highest quality, structure, and bio-activity: Freeze drying is the unequivocal choice, essential for high-value pharmaceuticals, biologicals, and premium foods.
- If your primary focus is efficient, cost-effective moisture removal for durable materials: Standard vacuum drying is the more practical and economical solution for bulk chemicals or food ingredients where some structural change is acceptable.
Understanding this core difference in water's physical state empowers you to select the precise drying method that aligns with your product's value and your operational goals.
Summary Table:
| Feature | Freeze Drying | Vacuum Drying |
|---|---|---|
| Water Removal Method | Sublimation (solid to gas) | Evaporation (liquid to gas) |
| Product Structure | Preserved, porous | Often shrinks or collapses |
| Heat Sensitivity | Excellent for delicate compounds | Risk of degradation |
| Process Cost | High | Lower |
| Processing Time | Slow (24-72+ hours) | Faster (hours) |
Unsure which drying method is right for your application? The experts at KINTEK are here to help. We specialize in providing the ideal lab equipment for your specific drying needs, whether you require the pristine preservation of freeze drying or the cost-effective efficiency of vacuum drying.
Let us help you make the right choice for your product's quality and your operational goals. Contact our team today for a personalized consultation!
Visual Guide
Related Products
- Benchtop Laboratory Freeze Dryer for Lab Use
- Benchtop Laboratory Vacuum Freeze Dryer
- Laboratory Sterilizer Lab Autoclave Pulse Vacuum Lifting Sterilizer
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
- Laboratory Test Sieves and Sieving Machines
People Also Ask
- Why is a laboratory freeze-drying system essential for fermentation biomass? Preserve Sample Integrity for Analysis
- Why is a laboratory vacuum freeze dryer essential for plant extracts? Preserve Bioactivity & Structure
- Why is a freeze dryer preferred for drying nickel nanoparticle precursors? Prevent Hard Agglomeration Now
- What is the function of a freeze dryer in the ice-templating process? Preserving Aligned Pore Scaffolds for LAGP
- What is the purpose of an evaporator? The Key Component That Creates Cooling



















