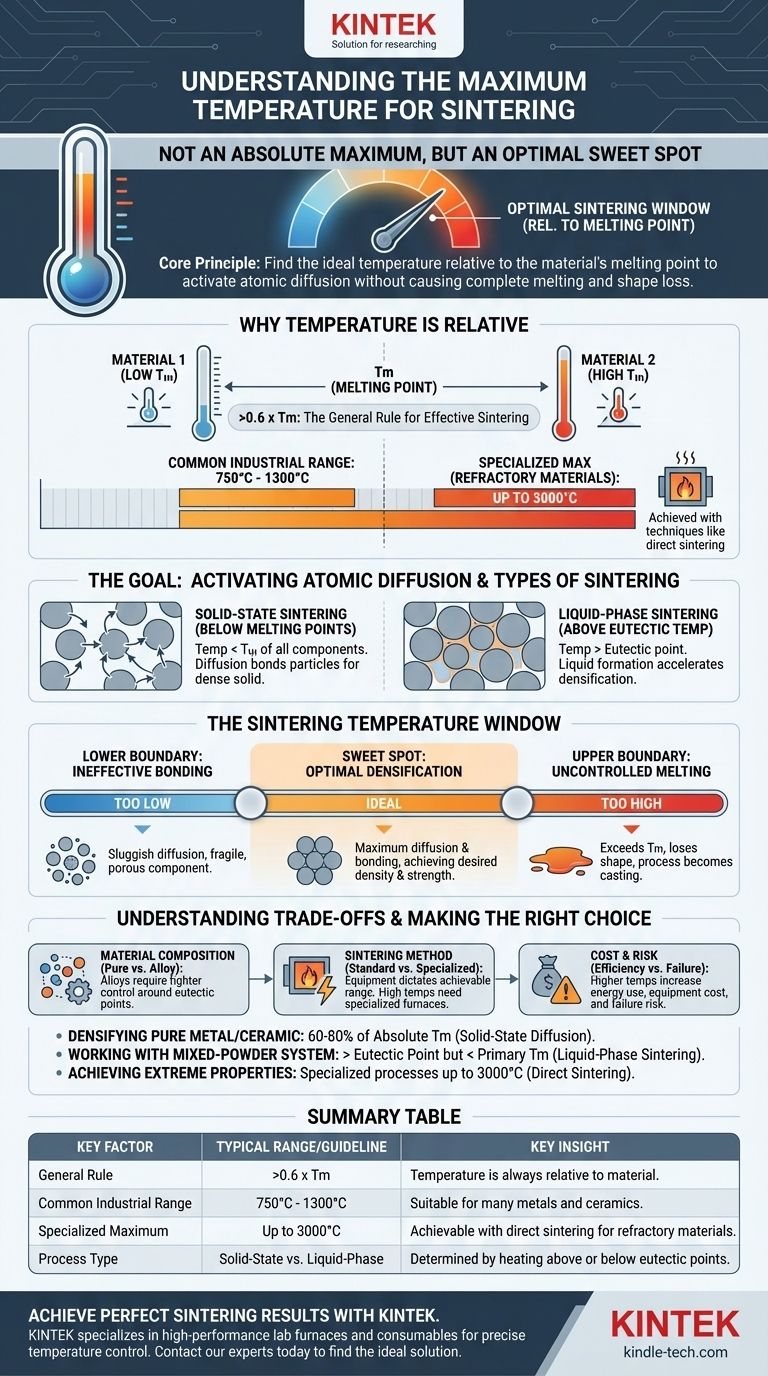
The maximum achievable temperature for sintering can be as high as 3000°C. However, this figure is only possible with specialized techniques like direct sintering for highly refractory materials. The appropriate sintering temperature is not a universal maximum but is fundamentally determined by the melting point of the specific material being processed.
The core principle of sintering is not to reach a maximum temperature, but to find the optimal temperature relative to the material's melting point. This "sweet spot" must be hot enough to activate atomic diffusion and bond particles together, but cool enough to prevent the component from losing its shape by melting completely.

Why Temperature Is Relative, Not Absolute
The concept of a single "maximum" temperature is misleading because sintering is a material-specific process. The correct temperature is always a function of the material's intrinsic properties.
The Rule of Thumb: The Melting Point (Tm)
As a general guideline, effective sintering requires temperatures greater than 0.6 times the material's absolute melting temperature (Tm). For many common industrial materials, this places the typical operating range between 750°C and 1300°C.
The Goal: Activating Atomic Diffusion
The primary purpose of heat in sintering is to give atoms enough energy to move. This process, known as diffusion, allows atoms to travel across the boundaries of adjacent powder particles, forming strong metallic or ceramic bonds that create a dense, solid part. Temperature is the main lever controlling the rate of this diffusion.
Solid-State vs. Liquid-Phase Sintering
Temperature control is also critical for determining the type of sintering that occurs. If the temperature stays below the melting point of all components, it is solid-state sintering.
If the material is a mix of powders, heating it above the eutectic temperature—the lowest point at which a liquid can form—initiates liquid-phase sintering. This small amount of liquid can dramatically accelerate densification.
The Sintering Temperature Window
Successfully sintering a component involves operating within a carefully defined temperature window. Deviating above or below this window leads to failed parts.
The Lower Boundary: Ineffective Bonding
If the temperature is too low, diffusion is sluggish or nonexistent. The powder particles will not bond effectively, resulting in a fragile, highly porous component that lacks the desired strength and density.
The Upper Boundary: Uncontrolled Melting
If the temperature is too high, it exceeds the material's melting point. Instead of bonding, the powder particles will simply melt into a puddle, and the component will lose its engineered shape. At this point, the process is no longer sintering; it has become casting.
The Sweet Spot: Optimal Densification
The ideal sintering temperature is the "sweet spot" that maximizes the rate of diffusion and particle bonding without risking structural collapse. This is where the part achieves maximum density and strength while retaining its intended form.
Understanding the Trade-offs
Choosing the right temperature involves balancing competing factors. It is a critical decision that impacts process efficiency, cost, and the final quality of the part.
Material Composition Is Key
Pure materials have a clear melting point, but alloys or powder mixtures are more complex. Their behavior depends on eutectic points and the melting temperatures of their various constituents, demanding much tighter process control.
Sintering Method Matters
The equipment directly influences the achievable temperature range. A standard furnace might operate up to 1300°C, which is sufficient for many metals. However, processing refractory materials like tungsten carbide or certain ceramics requires specialized high-temperature furnaces or methods like direct sintering, which uses high electrical current to reach temperatures up to 3000°C.
The Cost of Higher Temperatures
Pushing to higher temperatures is not always better. It requires more energy, more sophisticated (and expensive) furnaces, and creates a greater risk of part failure if not controlled with extreme precision. The goal is to use the lowest temperature that still achieves the desired material properties efficiently.
Making the Right Choice for Your Goal
The correct temperature strategy depends entirely on your material and desired outcome.
- If your primary focus is densifying a pure metal or ceramic: You will operate at a significant fraction (e.g., 60-80%) of its absolute melting temperature to drive solid-state diffusion.
- If your primary focus is working with a mixed-powder system (e.g., an alloy): You must carefully control the temperature to stay above the eutectic point for liquid-phase sintering but below the full melting point of the primary material.
- If your primary focus is achieving extreme properties with refractory materials: You will require specialized processes like direct sintering capable of reaching temperatures that approach 3000°C.
Ultimately, temperature is the most critical variable you can control to dictate the final density, strength, and structural integrity of a sintered component.
Summary Table:
| Key Factor | Typical Range / Guideline | Key Insight |
|---|---|---|
| General Rule | > 0.6 x Material's Melting Point (Tm) | Temperature is always relative to the specific material. |
| Common Industrial Range | 750°C - 1300°C | Suitable for many metals and ceramics. |
| Specialized Maximum | Up to 3000°C | Achievable with direct sintering for refractory materials. |
| Process Type | Solid-State vs. Liquid-Phase | Determined by heating above or below eutectic points. |
Achieve perfect sintering results for your specific materials and application. The right furnace and precise temperature control are critical for achieving the density, strength, and structural integrity your lab demands. KINTEK specializes in high-performance lab furnaces and consumables designed for a wide range of sintering temperatures and processes. Contact our experts today to discuss your sintering requirements and find the ideal solution for your laboratory's needs.
Visual Guide
Related Products
- Dental Porcelain Zirconia Sintering Ceramic Furnace Chairside with Transformer
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
- Vacuum Heat Treat and Sintering Furnace with 9MPa Air Pressure
- Spark Plasma Sintering Furnace SPS Furnace
- 600T Vacuum Induction Hot Press Furnace for Heat Treat and Sintering
People Also Ask
- Why is a high-temperature muffle furnace necessary for APTES-modified TiO2? Optimize Your Material Phase Transformation
- Are sintered parts conductive? Maximize Performance with Material and Density Control
- How is a high-temperature calcination furnace utilized in BZY20 Sol-gel? Achieve Pure Cubic Perovskite Phases
- What is the function of a high-temperature Box Furnace in rare earth oxide conversion? Enhance Chemical Reactivity
- Which insulator is used in muffle furnace? Choose the Right Refractory for Your Heat Needs
- How does a high-temperature muffle furnace function in NASICON calcination? Optimize Your Solid-State Synthesis
- What is the role of the high-temperature furnace in preparing BZCY ceramic? Master Phase Formation and Purity
- What is the working principle of laboratory muffle furnace? Achieve Contamination-Free High-Temperature Processing



















