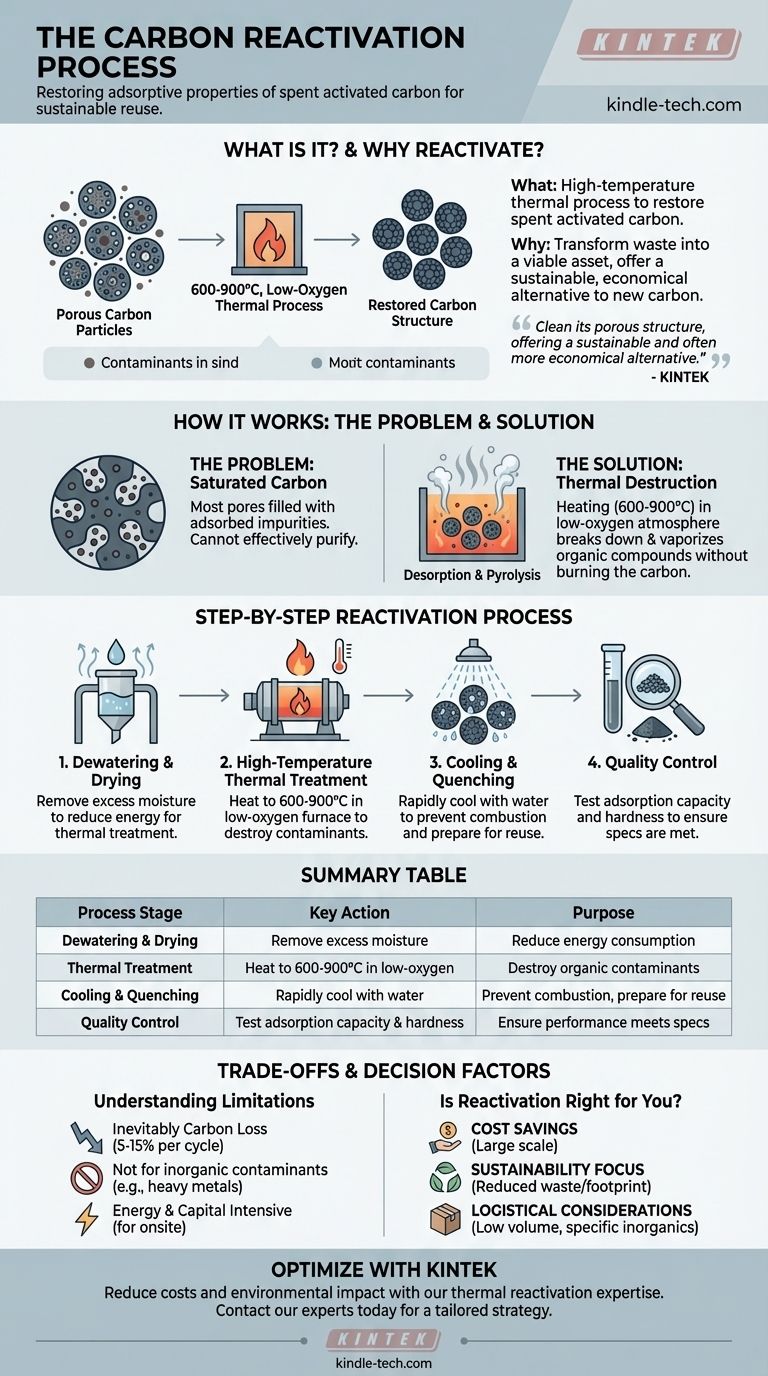
At its core, carbon reactivation is a high-temperature thermal process designed to restore the adsorptive properties of spent activated carbon. It involves heating the used carbon in a controlled, low-oxygen environment to between 600 and 900°C. This intense heat effectively burns off the organic contaminants that have been captured in the carbon's pores, regenerating it for reuse.
The central purpose of reactivation is to transform spent activated carbon from a waste product back into a viable asset. By cleaning its porous structure, the process offers a sustainable and often more economical alternative to continually purchasing new material.

How Carbon Reactivation Works
To understand reactivation, you must first understand what makes activated carbon "spent." Its effectiveness comes from a vast network of microscopic pores that trap, or adsorb, contaminants.
The Problem: Saturated Carbon
Activated carbon becomes "spent" or "saturated" when most of its available pores are filled with adsorbed impurities. At this point, it can no longer effectively purify water or air and must be replaced.
The Solution: Thermal Destruction
Reactivation reverses this process through thermal treatment in a furnace or kiln. The key is maintaining a low-oxygen atmosphere while heating the carbon to very high temperatures (600-900°C).
This environment causes the adsorbed organic compounds to undergo desorption and pyrolysis. They are broken down and vaporized, effectively burning them away from the carbon's surface without burning the carbon itself.
The Result: A Restored Structure
Once the contaminants are destroyed and driven off, the carbon's internal pore network is cleared. While a small percentage of the carbon's capacity is lost in each cycle, the vast majority of its adsorptive potential is restored, allowing it to be put back into service.
The Step-by-Step Reactivation Process
While the specific equipment can vary, the fundamental stages of reactivation are consistent. The process is far more controlled than simple carbonization.
Step 1: Dewatering and Drying
Spent carbon, especially from water treatment applications, is first dewatered and dried. This removes excess moisture, which reduces the energy required for the high-temperature heating stage.
Step 2: High-Temperature Thermal Treatment
The dried carbon is fed into a sealed reactivation furnace, often a rotary kiln. The system is heated to the target temperature range while the oxygen level is kept near zero to prevent the carbon from combusting. This is the critical stage where contaminants are destroyed.
Step 3: Cooling and Quenching
After exiting the furnace, the hot reactivated carbon must be cooled down carefully. This is typically done through a "quench" with water, which safely lowers its temperature and prevents it from combusting upon contact with oxygen in the open air.
Step 4: Quality Control
Finally, the reactivated carbon is tested to ensure it meets performance specifications for adsorption capacity, density, and hardness. It is then ready to be returned to the application.
Understanding the Trade-offs and Limitations
Reactivation is a powerful tool, but it is not a perfect or universally applicable solution. Understanding its limitations is crucial for making an informed decision.
Inevitable Carbon Loss
The process is not 100% efficient. With each reactivation cycle, a small amount of the carbon itself is lost, typically between 5% and 15%. This loss must be factored into economic calculations, as "make-up" carbon will be required.
Contaminant Compatibility
Reactivation is ideal for organic contaminants that can be thermally destroyed. It is not suitable for removing inorganic materials like heavy metals, as these will remain in the carbon and can accumulate to problematic levels over multiple cycles.
Energy and Capital Costs
Reactivation facilities are energy-intensive and require significant capital investment. For smaller operations, the cost of transporting the spent carbon to a third-party reactivation facility may be a more practical consideration than building an on-site system.
Is Reactivation the Right Choice for You?
Choosing between reactivation and disposal depends entirely on your operational scale, sustainability goals, and the nature of your contaminants.
- If your primary focus is cost savings at scale: Reactivation is almost always more economical than purchasing new carbon for large-volume applications.
- If your primary focus is sustainability: Reactivation dramatically reduces solid waste and lowers the carbon footprint associated with producing and transporting virgin material.
- If you handle low volumes or specific inorganics: The logistical complexity, high costs, or contaminant incompatibility may make simple disposal and replacement a more practical choice.
By weighing these factors, you can determine the most effective and responsible path for managing your activated carbon.
Summary Table:
| Process Stage | Key Action | Purpose |
|---|---|---|
| Dewatering & Drying | Remove excess moisture | Reduce energy consumption for thermal treatment |
| Thermal Treatment | Heat to 600-900°C in low-oxygen furnace | Destroy organic contaminants via pyrolysis |
| Cooling & Quenching | Rapidly cool with water | Prevent combustion and prepare carbon for reuse |
| Quality Control | Test adsorption capacity and hardness | Ensure performance meets specifications |
Optimize your activated carbon management with KINTEK.
If your laboratory or industrial process relies on activated carbon for purification, our expertise in thermal reactivation can help you significantly reduce costs and environmental impact. KINTEK specializes in providing high-performance lab equipment and sustainable solutions for managing consumables like activated carbon.
Contact our experts today to discuss how a tailored carbon reactivation strategy can enhance your operational efficiency and support your sustainability goals.
Visual Guide
Related Products
- Rotary Tube Furnace Split Multi Heating Zone Rotating Tube Furnace
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- Multi-zone Laboratory Tube Furnace
People Also Ask
- What is a rotary heat type furnace? The Ultimate Guide to Uniform Heating & Mixing
- What is the process of zirconium production? From Ore to High-Performance Metal & Ceramic
- At what temperature does wood pyrolysis begin? Control the Process for Biochar, Bio-Oil, or Syngas
- What is the temperature of a rotary hearth furnace? Find the Right Heat for Your Process
- How are tube furnaces classified based on the orientation of the tube? Choose the Right Design for Your Process



















