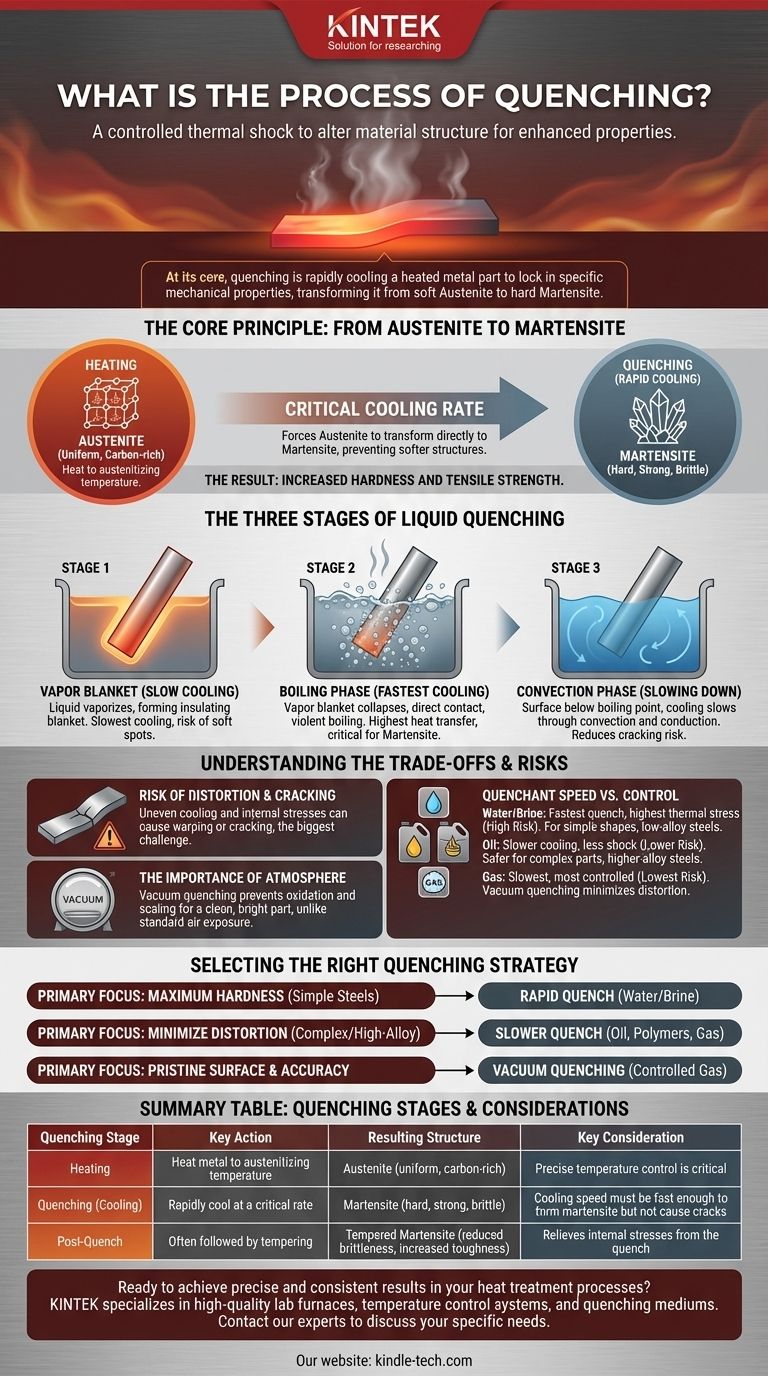
At its core, quenching is the process of rapidly cooling a heated metal part to lock in specific mechanical properties. This is not simply dunking hot metal in water; it is a controlled thermal shock engineered to intentionally alter the material's internal crystal structure, transforming it from a soft, malleable state at high temperature to a hard, strong state at room temperature.
Quenching is a carefully controlled metallurgical process, not just a cooling method. Its success depends entirely on cooling a workpiece at a precise rate—fast enough to trap a hard, strong crystal structure called martensite, but not so fast that it causes the part to crack or warp.

The Core Principle: From Austenite to Martensite
The entire purpose of quenching is to manage a phase transformation within the steel or alloy. This is achieved through a heat-and-cool cycle.
Heating to Form Austenite
First, the metal is heated to a specific, high temperature (known as the austenitizing temperature). At this temperature, the steel's crystal structure rearranges into a phase called austenite. In this state, the metal's structure is uniform and capable of dissolving carbon and other alloying elements into a solid solution.
The Critical Cooling Rate
Once the part is fully austenitized, it is cooled rapidly. This rapid cooling is the "quench." The speed is critical because it forces the austenite to transform directly into martensite, a very hard, strong, and brittle crystal structure. If the cooling is too slow, the metal will instead form softer structures like pearlite or bainite, defeating the purpose of the treatment.
The Result: Increased Hardness
The formation of martensite is what dramatically increases the hardness and tensile strength of the steel. This newly formed structure is highly strained and is the primary reason quenched components are exceptionally hard and wear-resistant.
The Three Stages of Liquid Quenching
When a hot part is submerged in a liquid like oil or water, the cooling process is not linear. It occurs in three distinct stages.
Stage 1: The Vapor Blanket (Slow Cooling)
Immediately upon submersion, the liquid touching the hot metal vaporizes, forming an insulating blanket of vapor around the part. Heat must radiate through this vapor layer, making this the slowest stage of cooling. An unstable vapor blanket can lead to uneven cooling and soft spots.
Stage 2: The Boiling Phase (Fastest Cooling)
As the surface cools slightly, the vapor blanket collapses, and the liquid quenchant makes direct contact with the part. This initiates violent boiling. The heat transfer rate during this nucleate boiling phase is extremely high and is the most critical part of the process for forming martensite.
Stage 3: The Convection Phase (Slowing Down)
Once the part's surface temperature drops below the boiling point of the liquid, boiling stops. Cooling continues at a much slower rate through convection and conduction into the surrounding liquid. This slower cooling helps reduce the risk of cracking.
Understanding the Trade-offs
Quenching is a balance of competing factors. Achieving maximum hardness often comes with significant risks that must be managed.
The Risk of Distortion and Cracking
The rapid cooling and martensitic transformation do not happen uniformly throughout the part. This creates immense internal stresses. If these stresses exceed the material's strength, the part can warp, distort, or even crack. This risk is the single biggest challenge in any quenching operation.
Quenchant Speed vs. Control
The choice of cooling medium (the "quenchant") is a trade-off between cooling power and control.
- Water/Brine: Provides the fastest quench but creates the highest thermal stress, increasing the risk of cracking. Best for simple shapes and low-alloy steels.
- Oil: Cools significantly slower than water, which lessens the thermal shock. This is a much safer choice for complex parts or higher-alloy steels where cracking is a concern.
- Gas: Used in vacuum quenching, gas (like nitrogen or argon) offers the slowest and most controlled quench. This provides maximum control and minimizes distortion.
The Importance of Atmosphere
In standard furnace quenching, the hot part is exposed to air, which causes oxidation and scaling on the surface. Processes like vacuum quenching heat the part in a controlled atmosphere or vacuum. This prevents any reaction with the surface, resulting in a clean, bright part that requires no post-process cleaning.
Selecting the Right Quenching Strategy
The ideal quenching process is dictated by the alloy being treated, the part's geometry, and the final properties required.
- If your primary focus is achieving maximum hardness in simple carbon steels: A rapid quench, often using water or brine, is necessary to exceed the critical cooling rate.
- If your primary focus is minimizing distortion and cracking in complex or high-alloy steels: A slower, more controlled quench using oil, specialized polymers, or gas is the safer and more effective choice.
- If your primary focus is a pristine surface finish and high-dimensional accuracy: Vacuum quenching with a controlled gas backfill is the superior method, as it prevents oxidation and offers the most gentle cooling profile.
Understanding these principles allows you to move beyond simply cooling a part and begin engineering its final performance characteristics.
Summary Table:
| Quenching Stage | Key Action | Resulting Structure | Key Consideration |
|---|---|---|---|
| Heating | Heat metal to austenitizing temperature | Austenite (uniform, carbon-rich) | Precise temperature control is critical |
| Quenching (Cooling) | Rapidly cool at a critical rate | Martensite (hard, strong, brittle) | Cooling speed must be fast enough to form martensite but not cause cracks |
| Post-Quench | Often followed by tempering | Tempered Martensite (reduced brittleness, increased toughness) | Relieves internal stresses from the quench |
Ready to achieve precise and consistent results in your heat treatment processes? The right laboratory equipment is crucial for controlling the quenching stages and achieving the desired material properties. KINTEK specializes in high-quality lab furnaces, temperature control systems, and quenching mediums designed for metallurgical applications. Whether you are working with simple carbon steels or complex alloys, our solutions help you manage the critical cooling rates necessary to form martensite while minimizing distortion and cracking.
Contact our experts today to discuss your specific quenching needs and discover how KINTEK's reliable lab equipment can enhance your material performance and process efficiency.
Visual Guide
Related Products
- Vertical Laboratory Tube Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Molybdenum Vacuum Heat Treat Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
People Also Ask
- How does an industrial high-temperature steam oxidation device ensure representative results? Simulating Reactor Safety
- What is heat treatment as used in metallic materials? Tailor Metal Properties for Superior Performance
- Why is a double vacuum resistance furnace recommended for magnesium recovery? Ensuring Safety & Stability
- What are the physical properties of sinter? Optimizing Strength, Reducibility & High-Temperature Performance
- Why use a vacuum drying oven for PEO/LiTFSI? Achieve High-Performance PEO/LLZTO Composite Solid Electrolytes
- Is it possible to braze cast iron? Yes, and It's Often the Safest Repair Method
- What temperature is ceramic sintering? Master the Heat for Strong, Dense Ceramics
- Why do we need vacuum for thermal evaporation? Ensure High-Quality Thin Film Deposition



















