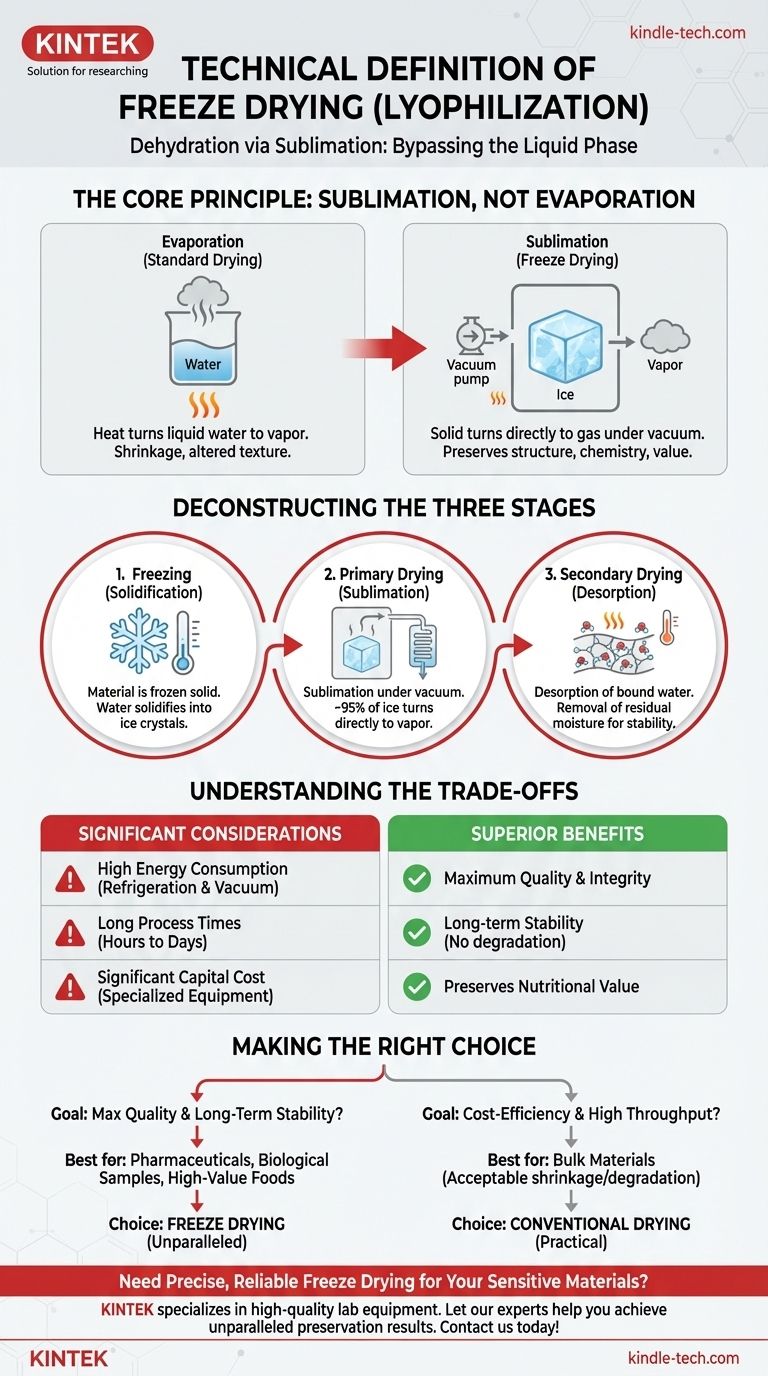
Technically, freeze drying is a dehydration process known as lyophilization. It works by first freezing a material to solidify its water content, then creating a powerful vacuum to force that solid ice to transform directly into a gas, completely bypassing the liquid phase. This unique method removes moisture while preserving the material's original structure, chemistry, and nutritional value.
At its core, freeze drying is not about simple dehydration but about sublimation. It is a controlled, three-stage process that removes water from a frozen state to prevent the structural collapse and chemical degradation common with heat-based drying.

The Core Principle: Sublimation, Not Evaporation
To truly understand freeze drying, you must distinguish it from conventional drying. The key difference lies in the physical state of the water as it is removed.
Bypassing the Liquid State
Standard dehydration relies on evaporation, where heat turns liquid water into vapor. This process often causes shrinkage, alters texture, and can destroy heat-sensitive nutrients and compounds.
Freeze drying, however, relies on sublimation. This is the physical process where a solid turns directly into a gas. By removing water as a vapor from a frozen solid, the product's underlying scaffold remains intact.
The Role of Vacuum Pressure
Sublimation is only possible under specific conditions of low temperature and low pressure. The vacuum created in a freeze dryer lowers the atmospheric pressure so significantly that water can transition from ice to vapor without ever melting into a liquid first.
Deconstructing the Three Stages of Lyophilization
The entire process is a meticulous, multi-stage operation designed to ensure complete and gentle dehydration.
Stage 1: Freezing (Solidification)
The first and most critical step is to lower the product's temperature until all the water within it is completely frozen solid. The speed of this freezing process is carefully controlled, as it can influence the size of the ice crystals, which in turn affects the speed of the subsequent drying stage and the quality of the final product.
Stage 2: Primary Drying (Sublimation)
Once frozen, the product is placed under a deep vacuum. A small, controlled amount of heat is then introduced to give the ice molecules just enough energy to sublimate.
The water vapor released is drawn away from the product and collected on an extremely cold condenser coil, which turns the vapor back into ice, effectively trapping it. This phase removes the majority (around 95%) of the water.
Stage 3: Secondary Drying (Desorption)
The final stage targets the last traces of unfrozen water molecules that are chemically bound to the material. The temperature is gradually raised and the vacuum pressure is often increased to break these bonds and remove this residual moisture. This final step is crucial for ensuring the product's long-term stability and preventing degradation during storage.
Understanding the Trade-offs
While freeze drying offers superior preservation, it is not without significant practical and economic considerations.
High Energy Consumption
Operating refrigeration systems to achieve deep freezes and running powerful vacuum pumps for extended periods is an energy-intensive process. This makes it one of the more costly methods of dehydration from an operational standpoint.
Long Process Times
Lyophilization is a slow, deliberate process. Depending on the material and its thickness, a complete cycle can take anywhere from several hours to multiple days. This low throughput can be a limiting factor for high-volume production.
Significant Capital Cost
The equipment required for freeze drying—the chamber, vacuum pumps, refrigeration units, and condensers—is highly specialized and represents a substantial capital investment compared to conventional ovens or dehydrators.
Making the Right Choice for Your Goal
Selecting a dehydration method depends entirely on your preservation requirements and operational constraints.
- If your primary focus is maximum quality and long-term stability: Freeze drying is the unparalleled choice for preserving sensitive pharmaceuticals, biological samples, or high-value foods where structural and chemical integrity is paramount.
- If your primary focus is cost-efficiency and high throughput: Conventional heat or air-drying is often more practical for bulk materials where some degree of shrinkage or nutritional degradation is acceptable.
Ultimately, understanding the principles of sublimation empowers you to select the right tool for achieving unparalleled preservation.
Summary Table:
| Stage | Process Name | Key Action | Result |
|---|---|---|---|
| 1 | Freezing | Material is frozen solid | Water solidifies into ice crystals |
| 2 | Primary Drying | Sublimation under vacuum | ~95% of ice turns directly to vapor |
| 3 | Secondary Drying | Desorption of bound water | Removal of residual moisture for stability |
Need precise, reliable freeze drying for your sensitive materials? KINTEK specializes in high-quality lab equipment, including freeze dryers designed to preserve the structural and chemical integrity of your pharmaceuticals, biological samples, and high-value food products. Let our experts help you achieve unparalleled preservation results. Contact us today to find the perfect solution for your laboratory's needs!
Visual Guide
Related Products
- Benchtop Laboratory Freeze Dryer for Lab Use
- Laboratory Sterilizer Lab Autoclave Herbal Powder Sterilization Machine for Plant
- Desktop Fast Laboratory Autoclave Sterilizer 35L 50L 90L for Lab Use
- Desktop Fast High Pressure Laboratory Autoclave Sterilizer 16L 24L for Lab Use
- Laboratory Hybrid Tissue Grinding Mill
People Also Ask
- What is the purpose of laboratory freeze drying? Preserve Sensitive Drugs & Biologics for Stability
- How does freeze drying benefit the cosmetics industry? Unlock Potent, Preservative-Free Formulas
- What role does a laboratory freeze dryer play in the synthesis of graphene-based electrocatalysts? Preserve 3D Structures
- What makes freeze-dried products advantageous for transport? Drastically Reduce Shipping Costs & Simplify Logistics
- What happens during the freezing phase of lyophilization? Master the Critical First Step for Product Integrity



















