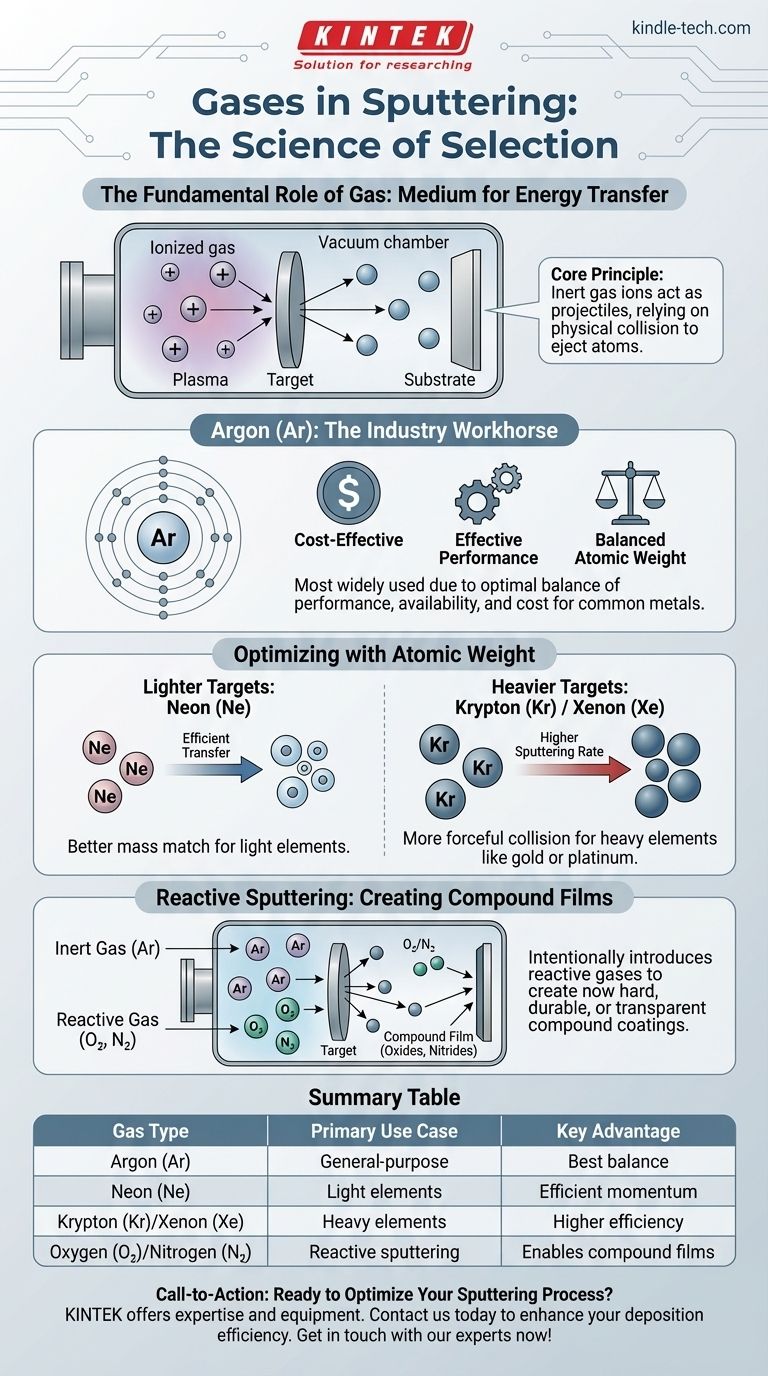
In the sputtering process, the primary gas used is Argon (Ar). While Argon is the most common choice due to its balance of cost and effectiveness, the selection of a gas is a critical process variable. Other noble gases like neon, krypton, or xenon are used for specific targets, and reactive gases like nitrogen or oxygen are introduced to create compound films.
The core principle is this: Sputtering relies on a purely physical collision to eject atoms from a target. Therefore, the ideal gas is chemically inert so it doesn't react with the material, and its atomic weight is matched to the target to ensure the most efficient transfer of momentum.

The Fundamental Role of Gas in Sputtering
To understand why a specific gas is chosen, we must first understand its function. The gas is not a reactant in the primary process; it is the medium for energy transfer.
Creating the Plasma Environment
The sputtering process begins by introducing a low-pressure gas into a vacuum chamber. An electric field is applied, which ionizes the gas atoms, stripping them of an electron and giving them a positive charge.
This cloud of ionized gas, ions, and free electrons is known as plasma.
The Principle of Momentum Transfer
The positively charged gas ions are accelerated by the electric field and slammed into the negatively charged "target"—the source material you wish to deposit.
Think of this as a subatomic game of pool. The gas ion is the cue ball, and the atoms of the target material are the billiard balls. The goal is to hit the target atoms with enough force to knock them loose so they can travel and coat a substrate.
Why Inert Noble Gases Are the Standard
The entire process hinges on the gas ions acting as clean, non-reactive projectiles. This is why noble gases from the far-right column of the periodic table are the standard choice.
The Need for Chemical Inertness
The primary requirement is that the gas does not chemically react with the target material. This ensures that the process is purely physical.
If the gas bonded with the target atoms, the resulting film would be an unintended compound, not the pure material you intended to deposit. Noble gases like argon have a full outer shell of electrons, making them extremely stable and non-reactive.
Argon: The Industry Workhorse
Argon is the most widely used sputtering gas because it provides the best balance of performance, availability, and cost. Its atomic weight is suitable for sputtering many of the most common metals and materials effectively.
Understanding the Trade-offs: Choosing the Right Gas
While argon is the default, optimizing the process for specific materials requires a more nuanced choice based on one key factor: atomic weight.
Matching Atomic Weight for Efficiency
For the most efficient momentum transfer—the most powerful "break" in our pool analogy—the mass of the cue ball (gas ion) should be as close as possible to the mass of the billiard ball (target atom).
Lighter Targets: Using Neon (Ne)
When sputtering very light elements, a heavy gas ion like argon can be inefficient, almost like hitting a ping pong ball with a bowling ball. A lighter noble gas like Neon provides a better mass match, leading to more efficient energy transfer for these specific targets.
Heavier Targets: Using Krypton (Kr) or Xenon (Xe)
Conversely, when sputtering heavy elements like gold or platinum, the relatively light argon ion is less effective. Using a heavier noble gas like Krypton or Xenon provides a much more forceful collision, significantly increasing the sputtering rate and efficiency.
The Exception: Reactive Sputtering
Sometimes, the goal is to intentionally create a compound film. In reactive sputtering, a reactive gas like oxygen or nitrogen is intentionally bled into the chamber along with the inert argon gas.
The argon ions still bombard the target, but as the target atoms travel to the substrate, they react with the oxygen or nitrogen. This allows for the deposition of hard, durable films like titanium nitride or transparent conductive films like indium tin oxide.
Making the Right Choice for Your Goal
The gas you select is not arbitrary; it is a tool to control the outcome of your deposition process.
- If your primary focus is general-purpose, cost-effective sputtering: Argon is the universal standard and the correct starting point.
- If your primary focus is the highest possible deposition rate for a heavy material: Krypton or Xenon are the superior choices, despite their higher cost.
- If your primary focus is sputtering a very light element: Neon may offer a more efficient and controlled process.
- If your primary focus is creating a specific compound film (e.g., an oxide or nitride): You will use a mixture of an inert gas (usually argon) and a specific reactive gas.
By understanding these principles, you can strategically select the right gas to achieve precise control over your thin-film deposition.
Summary Table:
| Gas Type | Primary Use Case | Key Advantage |
|---|---|---|
| Argon (Ar) | General-purpose sputtering | Best balance of cost, availability, and effectiveness for most metals |
| Neon (Ne) | Sputtering very light elements | Better mass match for efficient momentum transfer |
| Krypton (Kr) / Xenon (Xe) | Sputtering heavy elements (e.g., gold, platinum) | Higher sputtering rate and efficiency due to better mass match |
| Oxygen (O₂) / Nitrogen (N₂) | Reactive sputtering for compound films (e.g., oxides, nitrides) | Enables deposition of hard, durable compound coatings |
Ready to Optimize Your Sputtering Process?
Choosing the right sputtering gas is critical for achieving precise, high-quality thin films. Whether you're working with common metals, heavy elements, or need to create specific compound coatings, KINTEK has the expertise and equipment to support your laboratory's unique needs.
Contact us today to discuss your specific application and discover how our lab equipment and consumables can enhance your deposition efficiency and results. Get in touch with our experts now!
Visual Guide
Related Products
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Custom PTFE Teflon Parts Manufacturer for Culture Dish and Evaporation Dish
- Bomb Type Probe for Steelmaking Production Process
- Custom PTFE Teflon Parts Manufacturer for Air Valve Applications
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
People Also Ask
- What is a rotary vacuum evaporator? A Guide to Gentle & Efficient Solvent Removal
- Why do we use KBr in IR spectroscopy? Achieve Clear, High-Quality Solid Sample Analysis
- What are the advantages of using specialized supports in out-of-pack aluminizing? Achieve a Pristine Surface Finish
- How do you test a metal to determine its quality? Verify Mechanical & Chemical Properties for Your Application
- When and why does arcing occur? Understand the Physics to Prevent Costly Damage
- What is a drying furnace? Choose the Right Heating Method for Your Material
- What thickness is magnetron sputtering for coating? Achieve Precise, Functional Thin Films
- What is the burning temperature of a furnace? From 200°C to 3000°C, It Depends on Your Needs













