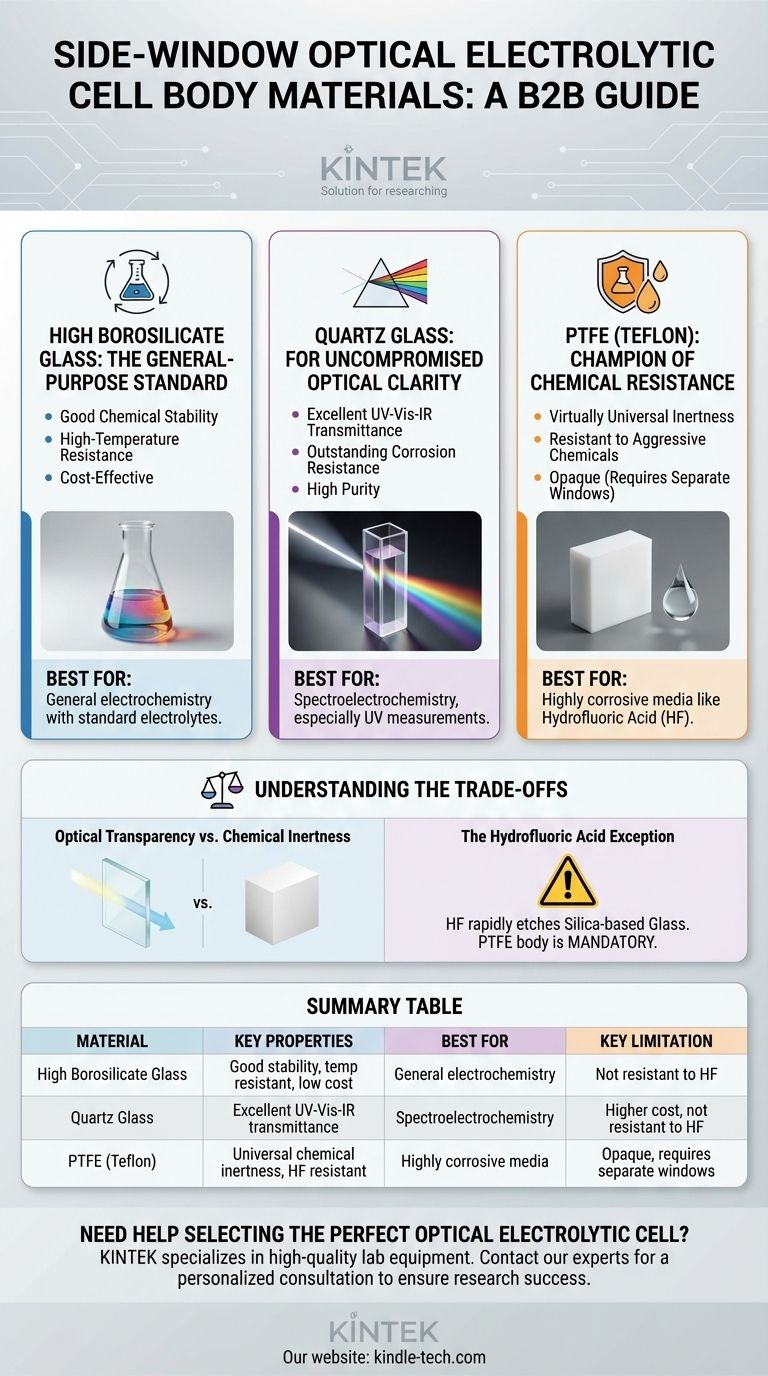
When selecting a side-window optical electrolytic cell, the body is most commonly constructed from one of three materials: high borosilicate glass, quartz glass, or Polytetrafluoroethylene (PTFE). Each material provides a distinct profile of chemical resistance, thermal stability, and optical properties, making the choice dependent on the specific demands of your experiment.
The selection of a cell body material is a critical experimental parameter, not a simple matter of availability. Your decision must be driven by the chemical nature of your electrolyte, the required temperature range, and, most importantly, the specific wavelengths of light you intend to measure.

A Closer Look at Common Cell Body Materials
Understanding the fundamental properties of each material is key to preventing experimental failure, sample contamination, or inaccurate measurements.
High Borosilicate Glass: The General-Purpose Standard
High borosilicate glass is the most common and cost-effective option for many applications. It serves as a reliable default for a wide range of electrochemical studies.
Its primary advantages are good chemical stability in the presence of most common acids, bases, and organic solvents, as well as robust high-temperature resistance.
Quartz Glass: For Uncompromised Optical Clarity
Quartz glass is a high-purity material chosen when optical measurements are the central goal of the experiment. Its performance here is unmatched.
The defining feature of quartz is its excellent light transmittance across the entire spectrum, from ultraviolet (UV) through visible and into the infrared (IR) range. It also possesses outstanding corrosion resistance, withstanding almost all acids.
PTFE (Teflon): The Champion of Chemical Resistance
Polytetrafluoroethylene, commonly known as PTFE or Teflon, is used when the chemical environment is too aggressive for any type of glass.
PTFE offers virtually universal corrosion resistance, making it inert to the most aggressive chemicals. It is the go-to material for experiments involving substances that would etch or destroy glass.
Understanding the Trade-offs
While each material excels in certain areas, none is perfect for every scenario. You must weigh their properties against their limitations.
Optical Transparency vs. Chemical Inertness
The most significant trade-off is between the optical clarity of glass and the superior chemical resistance of PTFE.
Quartz provides the best optical window but can be attacked by specific chemicals. PTFE is chemically indestructible but is opaque, meaning it can only be used for the cell body, requiring separate quartz or glass windows for the light path.
The Hydrofluoric Acid Exception
A critical limitation for both high borosilicate and quartz glass is their reaction with hydrofluoric acid (HF). This acid will rapidly etch and destroy any silica-based glass.
For any experiment involving HF, a PTFE body is mandatory. It is the only common material that can safely contain this highly corrosive substance without degrading.
Cost Considerations
Material choice also has a direct impact on cost. High borosilicate glass is the most economical option. Quartz is significantly more expensive due to its purity and manufacturing process. PTFE is also a premium-priced material, typically chosen out of necessity for its unique chemical resilience.
Making the Right Choice for Your Experiment
Your selection should be a deliberate decision guided by the specific demands of your research protocol.
- If your primary focus is general electrochemistry with standard electrolytes: High borosilicate glass provides the best balance of performance, visibility, and cost.
- If your primary focus is spectroelectrochemistry, especially in the UV range: Quartz is non-negotiable for its essential full-spectrum optical transparency.
- If your primary focus is working with highly corrosive media like hydrofluoric acid: A PTFE cell body is the only option that ensures the integrity and safety of your experiment.
Choosing the correct material is the foundational step toward acquiring reliable and reproducible electrochemical data.
Summary Table:
| Material | Key Properties | Best For | Key Limitation |
|---|---|---|---|
| High Borosilicate Glass | Good chemical stability, high-temperature resistance, cost-effective | General electrochemistry with standard electrolytes | Not resistant to hydrofluoric acid (HF) |
| Quartz Glass | Excellent UV-Vis-IR light transmittance, outstanding corrosion resistance | Spectroelectrochemistry, especially UV measurements | Higher cost, not resistant to HF |
| PTFE (Teflon) | Virtually universal chemical inertness, resistant to HF | Experiments with highly corrosive media (e.g., HF) | Opaque, requires separate optical windows |
Need help selecting the perfect optical electrolytic cell for your specific application?
At KINTEK, we specialize in high-quality lab equipment and consumables. Our experts can help you choose the right cell body material—whether it's high borosilicate glass, quartz, or PTFE—to ensure the accuracy, safety, and success of your electrochemical or spectroelectrochemical research.
Contact our team today for a personalized consultation and get the reliable performance your laboratory deserves.
Visual Guide
Related Products
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Electrolytic Electrochemical Cell with Five-Port
- PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
- Thin-Layer Spectral Electrolysis Electrochemical Cell
- Electrolytic Electrochemical Cell for Coating Evaluation
People Also Ask
- What is the structure of an H-type exchangeable membrane electrolytic cell? A Guide to Precise Electrochemical Separation
- What is the overall structure of the H-type double-layer optical water bath electrolytic cell? Precision Design for Controlled Experiments
- What optical features does the H-type electrolytic cell have? Precision Quartz Windows for Photoelectrochemistry
- What is the purpose of the double-layer structure in the H-type electrolytic cell? Achieve Precise Thermal Control
- What are the standard opening specifications for an H-type exchangeable membrane electrolytic cell? Asymmetrical Ports for Precise Electrochemistry



















