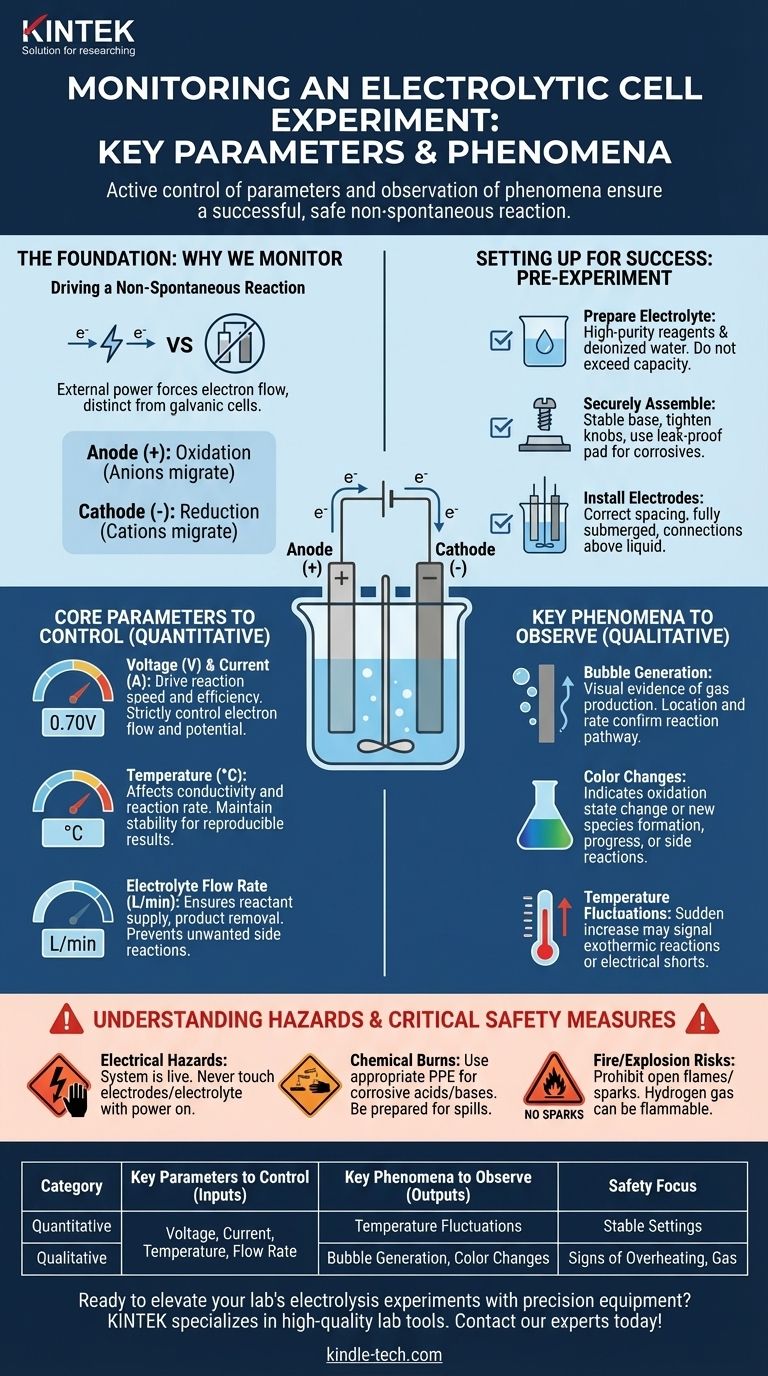
To ensure a successful experiment, you must actively control key parameters while simultaneously observing physical phenomena within the electrolytic cell. The primary parameters to control are the voltage, current, temperature, and electrolyte flow rate. Concurrently, you should closely watch for observable signs of the reaction, such as bubble formation on the electrodes, color changes in the solution, and any temperature variations.
Monitoring an electrolytic cell is not a passive act of data collection. It is an active process of steering a non-spontaneous reaction, where controlling quantitative parameters ensures the process runs as intended, while observing qualitative phenomena provides immediate feedback on the reaction's health and safety.

The Foundation: Why We Monitor
An electrolytic cell uses an external power source to drive a chemical reaction that would not otherwise occur. Understanding this core function is key to knowing what to monitor and why.
Driving a Non-Spontaneous Reaction
The entire system is powered by an external source, which forces electrons to flow in a specific direction. This is fundamentally different from a galvanic (voltaic) cell, which generates electricity from a spontaneous reaction.
The Role of Anode and Cathode
In an electrolytic cell, the terminology is distinct. The anode is the positive electrode where oxidation occurs, and the cathode is the negative electrode where reduction occurs. Anions (negative ions) migrate to the anode, while cations (positive ions) migrate to the cathode.
Setting Up for Success: Pre-Experiment Protocol
Effective monitoring begins before the power is even turned on. A proper setup minimizes variables and prevents catastrophic failures.
Prepare the Electrolyte
The purity of your electrolyte is critical. Always use high-purity chemical reagents and deionized or distilled water to prevent impurities from interfering with your primary reaction. When filling the cell, ensure you do not exceed its maximum capacity.
Securely Assemble the Cell
Place the electrolytic cell on a stable base and tighten any fixing knobs to ensure it remains stationary and vertical. If you are using a corrosive electrolyte, place a leak-proof pad underneath the cell as a crucial secondary containment measure.
Install the Electrodes
Install the electrodes in the vessel, ensuring they are correctly spaced and fully submerged in the electrolyte. However, the connection points of the electrode rods should remain above the liquid to prevent shorting or corrosion.
Core Parameters to Control
These are the quantitative inputs you will set and adjust to guide the reaction.
Voltage and Current
Voltage is the electrical potential driving the reaction, while current is the measure of the rate of electron flow. These two parameters are directly linked to the speed and efficiency of your electrolysis. They must be strictly controlled to ensure the reaction proceeds as planned.
Temperature
Temperature affects both the conductivity of the electrolyte and the rate of the electrochemical reaction. Maintaining a stable, controlled temperature is essential for obtaining reproducible results.
Electrolyte Flow Rate
In systems designed for continuous operation, the flow rate of the electrolyte is a critical parameter. It ensures that reactants are consistently supplied to the electrodes and that products are removed, preventing unwanted side reactions.
Key Phenomena to Observe
These qualitative observations are your real-time indicators of the reaction's status and health.
Bubble Generation at Electrodes
The formation of bubbles on an electrode surface is direct visual evidence that a gas is being produced. The location (anode or cathode) and rate of bubbling can help you confirm the reaction pathway.
Color Changes in the Solution
A change in the color of the electrolyte often signifies a change in the oxidation state of an ion or the formation of a new chemical species. This can be a primary indicator of your reaction's progress or the presence of an unintended side reaction.
Temperature Fluctuations
While you control the ambient temperature, you must also watch for unexpected temperature shifts within the cell. A sudden increase in temperature could indicate an unforeseen exothermic reaction or a dangerous electrical short.
Understanding Hazards and Critical Safety Measures
Your awareness during the experiment is the most important safety tool. Ignoring potential hazards can lead to serious consequences.
Electrical Hazards
The system is electrically live. Never touch the electrodes or electrolyte directly while the power supply is on to prevent severe electric shock.
Chemical Burns
Many electrolytes are corrosive acids or bases. Always handle them with appropriate personal protective equipment (PPE) and be prepared for spills.
Fire and Explosion Risks
Electrolysis can produce flammable gases, such as hydrogen. Strictly prohibit open flames or sparks near the electrolytic cell to prevent a fire or explosion.
Making the Right Choice for Your Goal
Your monitoring strategy should align with your experimental objective.
- If your primary focus is process efficiency and optimization: Concentrate on tightly regulating the quantitative parameters—voltage, current, temperature, and flow rate—to achieve the desired reaction rate.
- If your primary focus is identifying reaction products: Pay close attention to the observable phenomena, such as the location of bubble formation and any color changes, as these are direct clues to the chemical transformations taking place.
- If your primary focus is safety and data integrity: A comprehensive approach is mandatory, integrating meticulous setup, constant awareness of all hazards, and diligent monitoring of both parameters and phenomena.
By diligently controlling your inputs and observing the outputs, you transform the experiment from a passive event into a controlled and understood process.
Summary Table:
| Category | Key Parameters to Control | Key Phenomena to Observe |
|---|---|---|
| Quantitative Inputs | Voltage, Current, Temperature, Electrolyte Flow Rate | Temperature Fluctuations |
| Qualitative Outputs | - | Bubble Generation, Color Changes in Solution |
| Safety Focus | Stable electrical settings | Signs of overheating, rapid gas production |
Ready to elevate your lab's electrolysis experiments with precision equipment?
KINTEK specializes in high-quality lab equipment and consumables, providing the reliable tools you need for safe and accurate electrochemical research. From stable power supplies to durable electrodes and cells, we support your laboratory's success.
Contact our experts today to find the perfect solution for your experimental needs!
Visual Guide
Related Products
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- Customizable PEM Electrolysis Cells for Diverse Research Applications
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Multifunctional Electrolytic Electrochemical Cell Water Bath Single Layer Double Layer
- Electrolytic Electrochemical Cell for Coating Evaluation
People Also Ask
- What are the necessary steps to prepare an all-quartz electrolytic cell before an experiment? Ensure Accuracy and Safety
- What are the operational procedures and safety precautions during an experiment using an all-quartz electrolytic cell? Ensure Safety and Accuracy in Your Lab
- What materials are used to construct the all-quartz electrolytic cell? A Guide to Purity and Performance
- What is the proper procedure for post-experiment cleanup and storage of an all-quartz electrolytic cell? Ensure Longevity and Reproducibility
- What are the available volumes and dimensions for the all-quartz electrolytic cell? Find the Perfect Fit for Your Lab



















