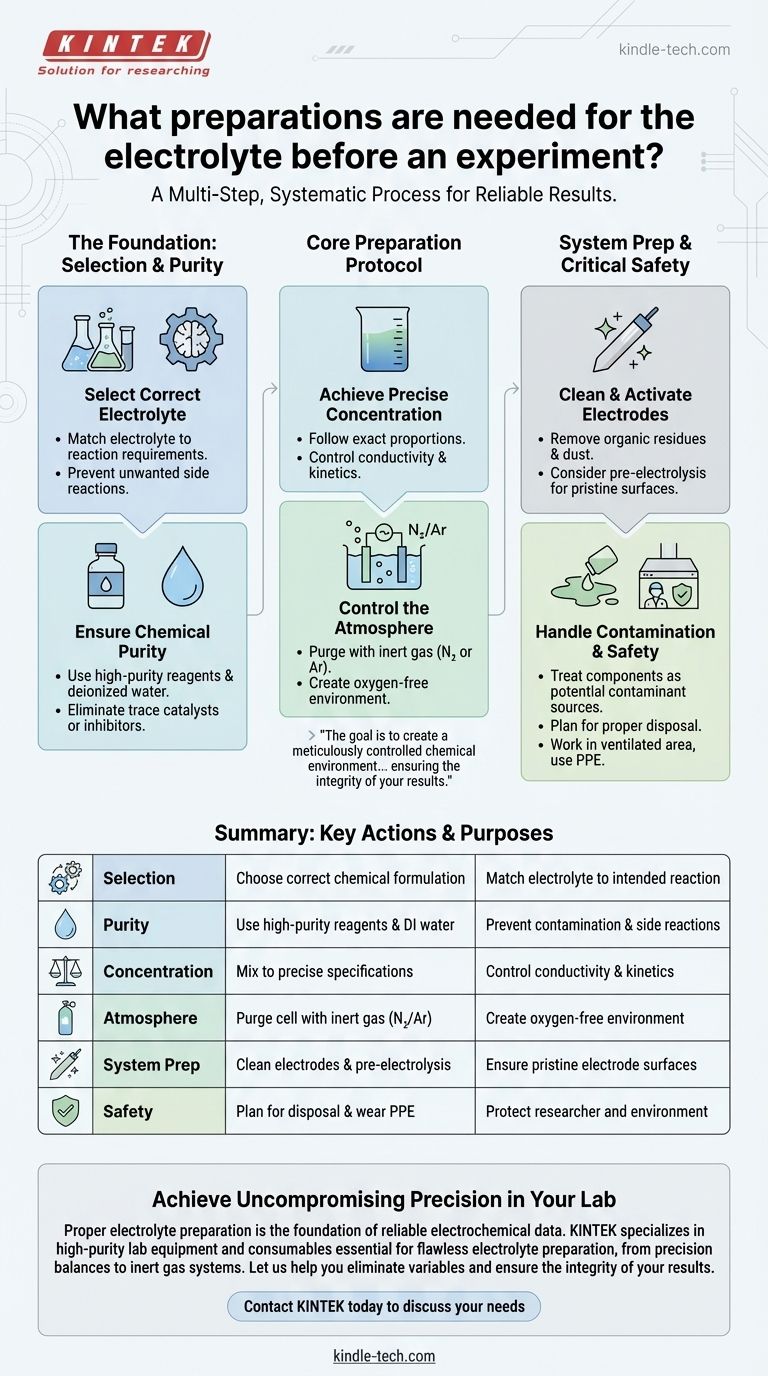
Proper electrolyte preparation is a multi-step, systematic process. It begins with selecting the correct chemical formulation for your specific reaction and verifying its purity. The core task involves mixing high-purity reagents and deionized water to a precise concentration, and for sensitive work, purging the electrochemical cell with an inert gas before introducing the solution.
The goal of electrolyte preparation is not simply to mix a solution. It is to create a meticulously controlled chemical environment where your intended reaction can proceed without interference from impurities or unwanted side reactions, thereby ensuring the integrity of your results.

The Foundation: Selecting the Right Electrolyte
Before any mixing occurs, you must choose the appropriate electrolyte. This decision is as critical as the experiment itself, as it defines the chemical environment for your reaction.
Matching the Electrolyte to Your Reaction
The electrolyte must be selected based on the specific requirements of your experiment. It needs to provide the necessary ions for conductivity without participating in the primary electrochemical reaction you intend to study.
Preventing Unwanted Side Reactions
A poorly chosen electrolyte can react at the electrode surfaces, creating byproducts or consuming charge. This leads to inaccurate measurements and complicates the analysis of your intended reaction.
The Core Preparation Protocol
Once the correct electrolyte is selected, its physical preparation demands precision and attention to detail to prevent contamination.
Ensuring Chemical Purity
Use only high-purity chemical reagents and deionized or distilled water. Even trace impurities from lower-grade materials can act as catalysts, inhibitors, or electroactive species, fundamentally altering your results.
Achieving Precise Concentration
Prepare the solution according to the exact proportions and methods dictated by your experimental design. The concentration of ions directly governs the solution's conductivity, the reaction kinetics, and the system's overall performance.
Controlling the Atmosphere
If your experiment is sensitive to air—particularly oxygen, which is easily reduced—you must remove it from your cell. Purge the vessel with an inert gas like high-purity nitrogen or argon before adding the electrolyte to create a controlled, oxygen-free environment.
Beyond the Liquid: Preparing the Full System
The electrolyte does not exist in isolation. Its interaction with the electrodes and the cell is paramount, requiring preparation of the entire system.
Cleaning and Activating Electrodes
The performance of the electrolyte is directly tied to the condition of the electrode surfaces. Before beginning, thoroughly clean the electrodes with deionized water or ethanol to remove any organic residues or dust.
Pre-Electrolysis for Ultimate Purity
For experiments demanding the highest precision, consider a final purification step known as pre-electrolysis. This involves running a small current through the system after filling it with the electrolyte to plate out or consume any remaining trace impurities, leaving a pristine electrode surface for your actual experiment.
Critical Considerations and Safety
A successful experiment is a safe and reproducible one. Ignoring potential pitfalls can invalidate your work and create hazards.
The Persistent Risk of Contamination
Contamination is the primary threat to reproducibility. Every component, including glassware, stir bars, and electrodes, must be treated as a potential source of impurities and cleaned accordingly.
Handling and Disposal Protocols
Your preparation is not complete until you have a plan for what to do after the experiment. Based on the electrolyte's chemical properties, this plan must include proper neutralization, recycling, or disposal methods to prevent environmental harm.
Personal and Environmental Safety
Electrolysis can generate high temperatures or hazardous gases. Always work in a well-ventilated area, such as a fume hood, and wear appropriate personal protective equipment (PPE). Be constantly aware of risks like electric shock, chemical burns, and poisoning.
Executing Your Preparation Flawlessly
Your preparation strategy should align directly with your experimental goals.
- If your primary focus is high-precision analytical measurement: You must prioritize extreme purity, precise concentration, and rigorous atmospheric control above all else.
- If your primary focus is bulk synthesis or educational demonstration: Your emphasis should be on achieving the correct concentration and ensuring robust safety protocols are in place.
Meticulous preparation is not a preliminary chore; it is the first and most critical step in generating trustworthy electrochemical data.
Summary Table:
| Preparation Step | Key Action | Purpose |
|---|---|---|
| Selection | Choose correct chemical formulation | Match electrolyte to intended reaction |
| Purity | Use high-purity reagents & deionized water | Prevent contamination & side reactions |
| Concentration | Mix to precise specifications | Control conductivity & reaction kinetics |
| Atmosphere | Purge cell with inert gas (N₂/Ar) | Create oxygen-free environment |
| System Prep | Clean electrodes & consider pre-electrolysis | Ensure pristine electrode surfaces |
| Safety | Plan for disposal & wear appropriate PPE | Protect researcher and environment |
Achieve Uncompromising Precision in Your Lab
Proper electrolyte preparation is the foundation of reliable electrochemical data. Whether your work demands high-purity analytical measurements or robust synthesis, having the right equipment is paramount.
KINTEK specializes in high-purity lab equipment and consumables essential for flawless electrolyte preparation. From precision balances for accurate weighing to inert gas purging systems and electrochemical cells, we provide the tools you need to create a meticulously controlled chemical environment.
Let us help you eliminate variables and ensure the integrity of your results.
Contact KINTEL today to discuss your specific laboratory needs and discover how our solutions can enhance your experimental workflow.
Visual Guide
Related Products
- Nickel Aluminum Tabs for Soft Pack Lithium Batteries
- Aluminum Foil Current Collector for Lithium Battery
- Ultra-Vacuum Electrode Feedthrough Connector Flange Power Electrode Lead for High-Precision Applications
- High Purity Zinc Foil for Battery Lab Applications
- Custom PTFE Teflon Parts Manufacturer for Non-Standard Insulator Customization
People Also Ask
- How do electrode spacing and monopolar parallel connections affect electrolytic performance? Optimize Energy & Efficiency
- What procedures should be followed during an experiment with a super-sealed electrolytic cell? Ensure Precision and Safety
- What are the recommended post-experiment procedures for cleaning and storing the thin-layer spectroelectrochemical cell?
- How should the H-type electrolytic cell be cleaned after use? Expert Maintenance for Pure Electrochemical Results
- What are the typical specifications for the volume and apertures of a side-window optical electrolytic cell? Key Specs for Your Spectroelectrochemistry
- What are the complete post-experiment procedures for a flat plate corrosion electrolytic cell? A Step-by-Step Guide to Reliable Results
- What are the electrode configuration requirements for a microfluidic E-cell? Precision Micro-Scale Corrosion Research
- What general precaution should be taken when handling the electrolytic cell? Ensure Safe and Accurate Lab Results



















