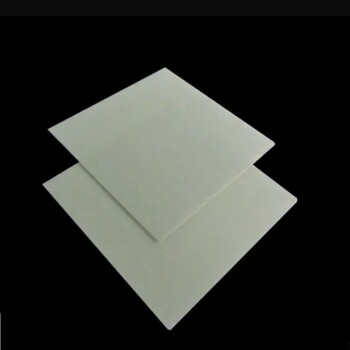
In short, steels with very low carbon content and most austenitic stainless steels cannot be hardened by conventional heat and quench methods. The ability of a steel to be hardened is fundamentally tied to its chemical composition, specifically its carbon content, which enables a critical change in its internal crystal structure.
The core principle is this: Heat treatment hardness is achieved by transforming a steel’s crystal structure into a hard, brittle phase called martensite. If a steel doesn't have enough carbon, or if its structure is stabilized by other elements, this transformation cannot occur.

The Defining Factor: Why Carbon is King
The Role of Carbon
Carbon is the single most important element for the conventional hardening of steel. It is the primary agent that allows the steel to form the martensitic structure required for high hardness.
Steels are classified by their carbon content. Low-carbon steels (often called mild steels) typically have less than 0.3% carbon. This is simply not enough carbon to achieve a significant hardening effect.
The Minimum Carbon Threshold
For a steel to be noticeably hardened by heat treatment, it generally needs a carbon content of at least 0.30% to 0.35%. Steels designed for high hardness, like tool steels, often have carbon levels of 1.0% or higher.
The Hardening Mechanism: A Tale of Two Structures
Heating to Create Austenite
When you heat a hardenable steel above a critical temperature (typically over 1400°F or 760°C), its crystal structure changes into a phase called austenite. In this state, the iron lattice can dissolve a significant amount of carbon.
Quenching to Trap Carbon
The magic happens during the quench—a rapid cooling in water, oil, or air. This sudden temperature drop doesn't give the carbon atoms time to move out of the crystal structure as they normally would during slow cooling.
The carbon becomes trapped, distorting the iron crystal lattice into a new, highly strained, and very hard structure known as martensite. This is the essence of hardening.
Steels That Resist Hardening (And Why)
Low-Carbon (Mild) Steels
As mentioned, steels with less than 0.3% carbon lack the necessary carbon atoms to effectively lock the crystal structure into martensite. When quenched, they largely return to their original soft state. These steels are valued for their ductility and weldability, not their hardness.
Austenitic Stainless Steels (304, 316)
This is the other major category. Austenitic stainless steels, like the common 304 and 316 grades, are specifically designed with high levels of nickel.
Nickel is an "austenite stabilizer." It forces the steel to remain in its soft, non-magnetic austenitic structure even at room temperature. Since the steel is already in the austenite phase and won't transform upon cooling, the martensitic reaction cannot be triggered.
It's important to note that these steels can be hardened, but through a different mechanism called work hardening (or strain hardening), which occurs by mechanically deforming the metal (e.g., bending or rolling).
Ferritic Stainless Steels (e.g., 430)
This group of stainless steels has very low carbon content and a crystal structure (ferrite) that does not transform into austenite when heated. With no austenite to start from, the martensitic transformation is impossible.
Understanding the Trade-offs
Hardness vs. Ductility
There is no "free lunch" in metallurgy. The martensitic structure that provides incredible hardness and wear resistance also makes the steel very brittle.
This is why hardened parts are almost always tempered after quenching. Tempering is a low-temperature heat treatment that reduces some hardness but restores crucial toughness and ductility, preventing the part from shattering in service.
Weldability and Machinability
Steels that are easily hardened (i.e., higher carbon and alloy content) are generally more difficult to weld. The rapid heating and cooling cycles of welding can create brittle zones near the weld, leading to cracking.
Similarly, high-carbon steels are much more difficult to machine in their hardened state. Machining is typically performed when the steel is in its soft, annealed condition before the final heat treatment.
Making the Right Choice for Your Application
Selecting the correct steel requires understanding your primary goal. The inability to harden a material is not a flaw if hardness is not the required property.
- If your primary focus is maximum hardness and wear resistance: Choose a high-carbon steel or a dedicated tool steel (like A2 or D2) designed for heat treatment.
- If your primary focus is corrosion resistance and ductility: Choose an austenitic stainless steel (like 304) and accept that its hardness comes from work hardening, not heat treatment.
- If your primary focus is cost-effective fabrication and excellent weldability: Choose a low-carbon steel (like A36 or 1018) and understand that it cannot be significantly through-hardened.
Understanding a steel's fundamental properties is the first step toward successful engineering and design.
Summary Table:
| Steel Type | Carbon Content | Hardening Mechanism | Key Characteristics |
|---|---|---|---|
| Low-Carbon (Mild) Steel | < 0.3% | Cannot be hardened | High ductility, excellent weldability |
| Austenitic Stainless Steel (304, 316) | Low | Work hardening only | Corrosion-resistant, non-magnetic |
| Ferritic Stainless Steel (e.g., 430) | Very Low | Cannot be hardened | Good corrosion resistance, magnetic |
| High-Carbon/Tool Steel | > 0.35% | Heat & quench hardening | High hardness, wear resistance |
Need the right steel for your specific application? Whether you require high-hardness tool steels for demanding tools or corrosion-resistant stainless steels for lab equipment, KINTEK has the expertise and materials to meet your laboratory needs. Our team can help you select the perfect steel based on your requirements for hardness, ductility, weldability, and corrosion resistance.
Contact us today to discuss your project and discover how KINTEK's lab equipment and consumables can enhance your operational efficiency and results!
Visual Guide
Related Products
- Custom PTFE Teflon Parts Manufacturer for PTFE Tweezers
- Vacuum Bellows for Efficient Connection and Stable Vacuum in High-Performance Systems
- Custom PTFE Teflon Parts Manufacturer for PTFE Mesh F4 Sieve
- Glassy Carbon Sheet RVC for Electrochemical Experiments
- Stainless Steel Quick Release Vacuum Chain Three-Section Clamp
People Also Ask
- What is the role of high-temperature sintering furnaces in 3D solid electrolyte frameworks? Achieve Peak Performance
- Can heat transfer occur in a vacuum? Yes, through radiation, the only way heat travels in space.
- What can I use for annealing steel? Master the Tools for Perfect Heat Treatment
- Why is a high-precision heat treatment furnace necessary for maraging steel? Ensure Peak SLM Part Performance
- How is hydrogen produced in pyrolysis? A Low-Carbon, Energy-Efficient Path to Clean Hydrogen
- How does a vacuum drying oven benefit Al2O3-TiCN/Co-Ni slurry processing? Protect Material Integrity & Purity
- What are the advantages of torch brazing? Discover the Superior Control of Modern Brazing
- What is the use of a calciner? A Guide to High-Temperature Material Transformation