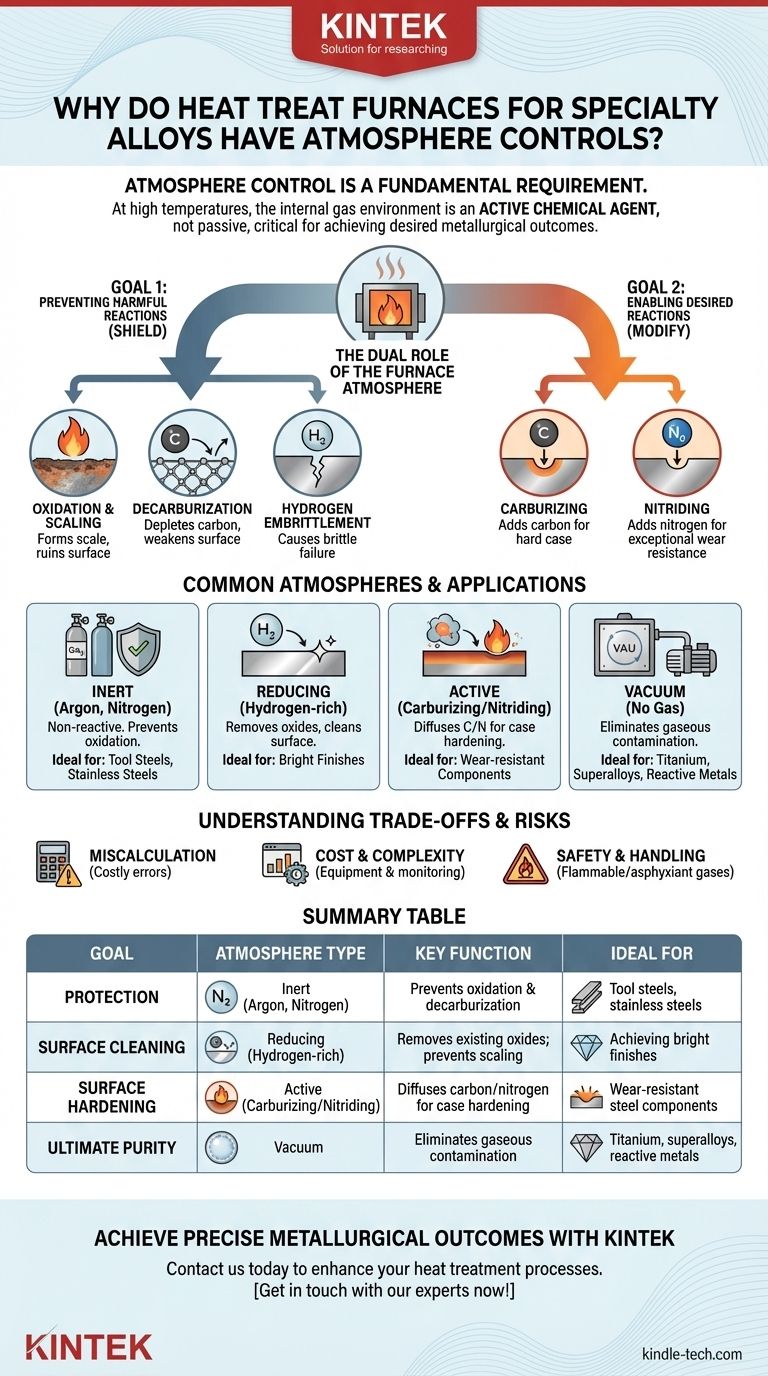
Atmosphere control is a fundamental requirement in heat treating specialty alloys because, at high temperatures, the furnace's internal gas environment is not passive. This atmosphere becomes a highly active chemical agent that can either protect the alloy from damage, like oxidation, or be used intentionally to alter its surface chemistry and achieve specific properties like enhanced hardness.
The core principle to understand is that the furnace atmosphere is an active ingredient in the heat treatment process. For expensive and sensitive specialty alloys, controlling this chemical environment is just as critical as controlling the temperature to achieve the desired metallurgical outcome.

The Dual Role of the Furnace Atmosphere
At the elevated temperatures required for heat treatment, metals are highly reactive. The gas surrounding the part—the atmosphere—dictates which chemical reactions will occur on its surface. This control is exercised for two primary reasons: protection and modification.
The First Goal: Preventing Harmful Reactions
The most basic function of atmosphere control is to shield the alloy from unwanted chemical changes that degrade its properties.
Oxidation and Scaling: In the presence of oxygen (even from plain air), hot metal surfaces will rapidly oxidize, forming a layer of scale. This changes the part's dimensions, ruins the surface finish, and can deplete critical alloying elements from the surface.
Decarburization: For carbon-based steels, an improperly controlled atmosphere can actually pull carbon atoms out of the alloy's surface. This leaves a soft, weak outer layer that severely compromises the material's strength and fatigue life.
Hydrogen Embrittlement: Certain atmospheres, particularly those with high moisture content, can introduce atomic hydrogen into the grain structure of some alloys. This can lead to a severe loss of ductility and premature, brittle failure under stress.
The Second Goal: Enabling Desired Reactions
Beyond simple protection, atmosphere control allows for the intentional modification of an alloy's surface, a process known as case hardening.
Carburizing: This process uses a carbon-rich atmosphere (typically containing carbon monoxide and hydrocarbons) to diffuse carbon atoms into the surface of a low-carbon steel. The result is an alloy with a hard, wear-resistant surface (case) and a tough, ductile interior (core).
Nitriding: Similarly, nitriding uses a nitrogen-rich atmosphere (often from dissociated ammonia) to diffuse nitrogen into the surface. This forms extremely hard nitride compounds, creating exceptional wear resistance and surface hardness.
Common Atmospheres and Their Applications
The choice of atmosphere depends entirely on the alloy being treated and the desired outcome.
Inert Atmospheres
Gases like Argon and Nitrogen are used to create a non-reactive environment. Their purpose is purely protective, displacing oxygen to prevent oxidation and decarburization. This is common for tool steels and stainless steels where preserving the existing chemistry is the only goal.
Reducing Atmospheres
Atmospheres rich in Hydrogen and Carbon Monoxide are considered "reducing." Not only do they prevent oxidation, but they can actively react with and remove (or "reduce") light surface oxides that may already be present on the part.
Active (Carburizing) Atmospheres
Generated by reacting air and a hydrocarbon gas, endothermic gas is a common active atmosphere. It is carefully balanced to have a specific "carbon potential," allowing it to add a precise amount of carbon to the steel's surface for case hardening.
Vacuum
A vacuum furnace achieves atmosphere control by removing it entirely. Pumping the chamber down to a near-perfect vacuum provides the ultimate protection from gaseous impurities, making it ideal for highly reactive materials like titanium, refractory metals, and nickel-based superalloys.
Understanding the Trade-offs and Risks
While essential, implementing atmosphere control introduces its own set of complexities and potential failure points.
The Consequence of Miscalculation
The most significant risk is getting the atmosphere's chemistry wrong. An atmosphere intended to be protective can become decarburizing if its carbon potential is lower than the steel's, ruining the parts. This can be a costly error with specialty alloys.
Cost and Complexity
Generating and monitoring specific gas mixtures requires significant investment in equipment. This includes gas generators, flow meters, and sophisticated sensors (like oxygen probes and dew point analyzers) to ensure the atmosphere remains within tight specifications throughout the heating cycle.
Safety and Handling
Many controlled atmospheres involve gases that are either flammable (hydrogen, carbon monoxide, natural gas) or are asphyxiants (nitrogen, argon). Proper safety protocols, ventilation, and monitoring are critical to safe furnace operation.
Making the Right Choice for Your Goal
The selection of a furnace atmosphere is a direct function of your material and your engineering objective.
- If your primary focus is maximum protection for highly reactive alloys (e.g., titanium, superalloys): A vacuum furnace is the superior choice to prevent any gaseous contamination.
- If your primary focus is creating a hard, wear-resistant surface on steel: You need an active atmosphere for carburizing or nitriding to add the necessary elements.
- If your primary focus is preventing scale and decarburization on tool steels: An inert atmosphere of nitrogen or argon provides effective and reliable protection.
- If your primary focus is a clean, bright finish on common steels: A reducing atmosphere containing hydrogen will prevent oxidation and can help clean the part's surface.
Ultimately, mastering atmosphere control is what transforms a simple heating operation into a precise and repeatable manufacturing process.
Summary Table:
| Goal | Atmosphere Type | Key Function | Ideal For |
|---|---|---|---|
| Protection | Inert (Argon, Nitrogen) | Prevents oxidation & decarburization | Tool steels, stainless steels |
| Surface Cleaning | Reducing (Hydrogen-rich) | Removes existing oxides; prevents scaling | Achieving bright finishes |
| Surface Hardening | Active (Carburizing/Nitriding) | Diffuses carbon/nitrogen for case hardening | Wear-resistant steel components |
| Ultimate Purity | Vacuum | Eliminates gaseous contamination | Titanium, superalloys, reactive metals |
Achieve precise metallurgical outcomes for your specialty alloys with KINTEK.
Your heat treatment process is only as good as the environment you create. Whether you need to protect high-value components from oxidation, perform precise case hardening, or work with highly reactive materials like titanium, the right furnace atmosphere is critical.
KINTEK specializes in lab equipment and consumables, providing reliable solutions for all your laboratory needs. Our expertise ensures you have the right tools to control your furnace atmosphere accurately, safeguarding your material properties and achieving repeatable, high-quality results.
Contact us today to discuss your specific application and discover how our solutions can enhance your heat treatment processes.
Get in touch with our experts now!
Visual Guide
Related Products
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- Vertical Laboratory Tube Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
People Also Ask
- What role does an inert gas-protected heating device play in 2024Al/Gr/SiC composite manufacturing?
- What role does the reducing protective gas play in Cu-SiOC hybrid ceramics? Ensure Conductivity via Active Reduction
- Why Use a Precise Atmosphere High-Temperature Furnace for Zirconia Research? Verify Oxygen Self-Diffusion Theories
- What is the use of annealing process in metal industry? Relieve Stress and Increase Ductility for Manufacturing
- Why use a tube atmosphere furnace with inert gas for catalyst calcination? Protect Active Sites from Contamination
- What is the nominal gas composition produced by different endothermic generation methods? Optimize Your Heat Treatment
- What is a controlled atmosphere furnace? Achieve Purity and Precision in High-Temp Processing
- What role does a high-temperature atmosphere furnace play in 3D Graphene Oxide production? Unlock Advanced Exfoliation



















