In modern steelmaking, argon is used as a multipurpose tool to protect, purify, and homogenize molten steel. Its primary function stems from its chemical inertness, which prevents the liquid metal from reacting with atmospheric oxygen and nitrogen, but it is also used physically to stir the melt and remove dissolved gases and impurities.
Argon is not merely a passive shield in steelmaking; it is an active instrument. It allows producers to control the steel's final chemistry and cleanliness with high precision, transforming a brute-force process into a sophisticated manufacturing science.

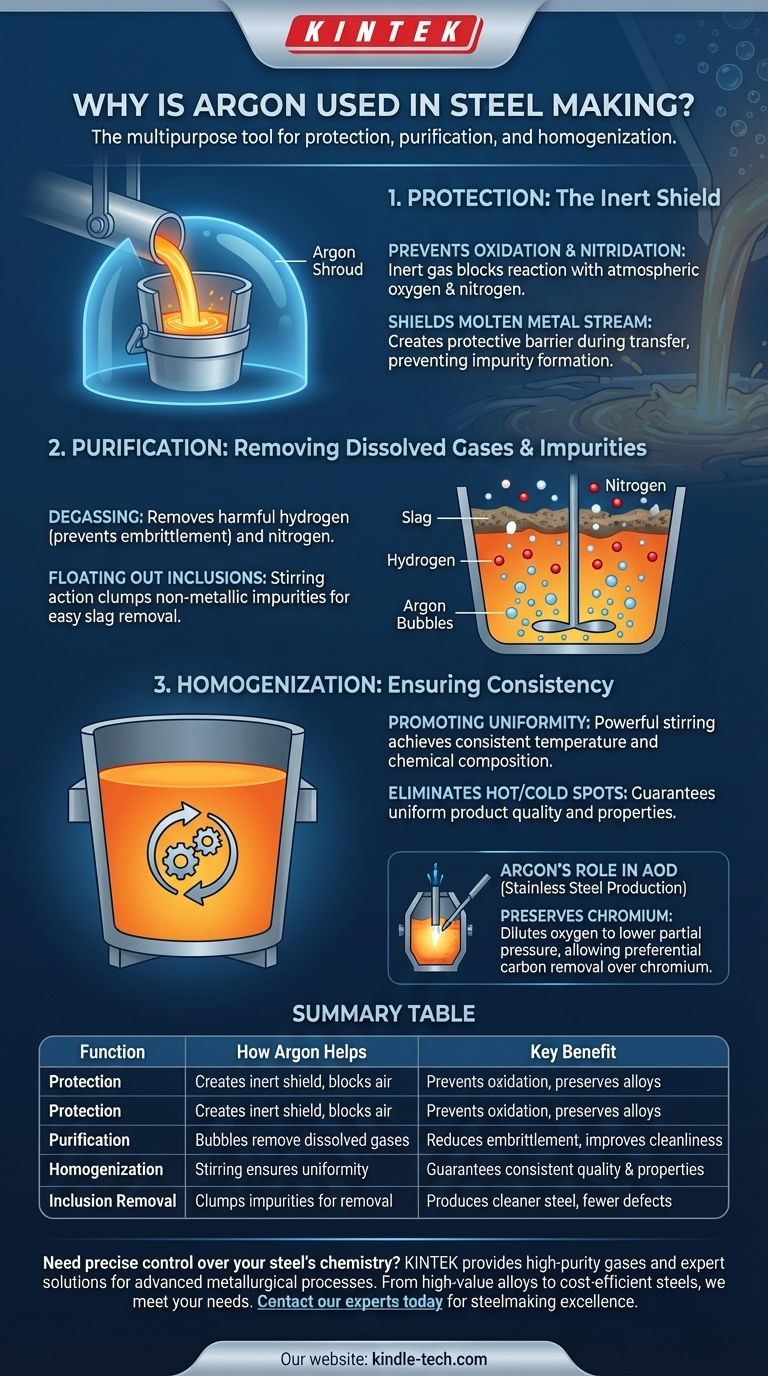
The Core Principle: Why Inertness Matters
The fundamental challenge in steelmaking is that molten steel, at temperatures exceeding 1600°C (2900°F), is extremely reactive. Contact with the ambient air can ruin a batch of steel in seconds.
Preventing Oxidation and Nitridation
At high temperatures, the iron and valuable alloying elements in the steel will readily react with oxygen and nitrogen from the air. This forms oxides and nitrides, which are non-metallic impurities.
These impurities become trapped in the steel as it solidifies, creating weak points that can lead to brittleness, cracking, and poor performance in the final product. Argon, being a noble gas, is almost completely non-reactive and displaces the air, creating a protective atmosphere.
Shielding the Molten Metal Stream
Any time molten steel is transferred from one vessel to another—for example, from the furnace to a ladle, or from a ladle into a continuous caster—it is exposed.
This process, known as shrouding or curtaining, uses a flow of argon to create an invisible, protective barrier around the stream of liquid steel. This prevents air from coming into contact with the metal during this highly vulnerable stage.
Argon as a Physical Tool: Stirring and Purification
Beyond its protective chemical properties, the physical act of bubbling argon gas through molten steel is critical for refining the product. This is done in vessels like the Ladle Metallurgy Furnace (LMF).
Promoting Homogeneity
A ladle of steel can contain hundreds of tons of liquid metal. Bubbling argon from the bottom creates a powerful, continuous stirring action.
This stirring ensures both temperature and chemical composition are uniform throughout the entire melt. It eliminates hot or cold spots and ensures that added alloys are perfectly mixed, guaranteeing a consistent product.
Removing Dissolved Gases
Molten steel can dissolve harmful gases, particularly hydrogen. As the steel cools, the solubility of hydrogen decreases, and it can form internal voids and flakes, a defect known as hydrogen embrittlement.
As argon bubbles rise through the melt, dissolved hydrogen and nitrogen diffuse into the bubbles and are carried harmlessly to the surface. This degassing process is crucial for producing high-quality, clean steel.
Floating Out Inclusions
The stirring motion created by the argon bubbles also helps small, solid impurities (the oxides and nitrides mentioned earlier) collide and clump together.
These larger, agglomerated inclusions are more buoyant and float to the surface more easily. There, they are absorbed into the slag layer, a liquid blanket of impurities that can be skimmed off.
Argon's Critical Role in AOD Converters
For stainless steel production, the Argon Oxygen Decarburization (AOD) process is essential, and argon is its namesake.
Stainless steel contains high levels of expensive chromium, which must be protected. To remove excess carbon, pure oxygen is blown into the melt, but this would also rapidly oxidize the chromium.
By diluting the oxygen with argon, the partial pressure of oxygen is lowered. This allows carbon to be preferentially oxidized and removed as carbon monoxide gas, while preserving the valuable chromium in the steel.
Understanding the Trade-offs
While argon is highly effective, its use is governed by a balance of quality requirements and cost.
The Cost Factor
Argon is produced by the fractional distillation of liquid air, an energy-intensive and expensive process. Its cost is a significant operational consideration for any steel mill.
Nitrogen as a Cheaper Alternative
Nitrogen is roughly ten times cheaper than argon. For many common carbon steel grades where nitrogen is not considered a harmful impurity (and may even be a desired alloying element), it is often used for stirring and shrouding instead of argon.
The Choice Is Grade-Dependent
The decision to use argon, nitrogen, or a mix of the two is dictated by the final steel grade. For high-value stainless steels or specialty alloys where purity is paramount, argon is non-negotiable. For commodity-grade structural steel, nitrogen is often sufficient.
Making the Right Choice for Your Goal
The strategy for using argon depends entirely on the type of steel being produced and the quality demanded.
- If your primary focus is high-value, specialty steels: Argon is essential to protect expensive alloys like chromium and achieve the ultra-low levels of impurities required.
- If your primary focus is cost-effective commodity steel: Nitrogen is often the preferred gas for general stirring and shrouding, with argon reserved only for the most critical steps.
- If your primary focus is superior mechanical properties: Argon-driven degassing is a key process for removing hydrogen to prevent embrittlement and create the cleanest possible steel.
Ultimately, the controlled use of argon elevates steelmaking from a simple melting process to a precise engineering discipline.
Summary Table:
| Function | How Argon Helps | Key Benefit |
|---|---|---|
| Protection | Creates an inert shield to prevent reaction with air | Prevents oxidation and nitridation, preserving alloying elements |
| Purification | Bubbles through melt to remove dissolved hydrogen/nitrogen | Reduces embrittlement and improves steel cleanliness |
| Homogenization | Stirring action ensures uniform temperature and chemistry | Guarantees consistent quality and properties in the final product |
| Inclusion Removal | Helps clump impurities for easy removal into slag | Produces cleaner steel with fewer defects |
Need precise control over your steel's chemistry and quality? KINTEK provides the high-purity gases and expert solutions essential for advanced metallurgical processes. Whether you're producing high-value alloys or optimizing cost-efficiency for commodity steels, our lab equipment and consumables are designed to meet your specific needs. Contact our experts today to discuss how we can support your steelmaking excellence.
Visual Guide
Related Products
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- Vacuum Heat Treat Sintering Brazing Furnace
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
People Also Ask
- What is an inert condition? A Guide to Preventing Fires and Explosions
- What is meant by inert atmosphere? A Guide to Preventing Oxidation & Ensuring Safety
- What gases are used in inert atmospheres? Choose the Right Gas for Non-Reactive Environments
- What provides an inert atmosphere? Achieve Safety and Purity with Nitrogen, Argon, or CO2
- Why nitrogen is used in annealing furnace? To prevent oxidation and decarburization for superior metal quality



















