Graphite's remarkably high melting point is a direct result of its unique atomic structure. To melt graphite, you must break the incredibly strong covalent bonds that connect its carbon atoms into extensive, flat sheets. This process requires a massive amount of energy, leading to a melting point around 3,600°C (6,500°F), which is even higher than that of diamond.
The immense energy needed to break the strong covalent bonds within graphite's carbon layers is the sole reason for its high melting point. The weak forces between these layers, which allow them to slide, are irrelevant to the melting process itself.

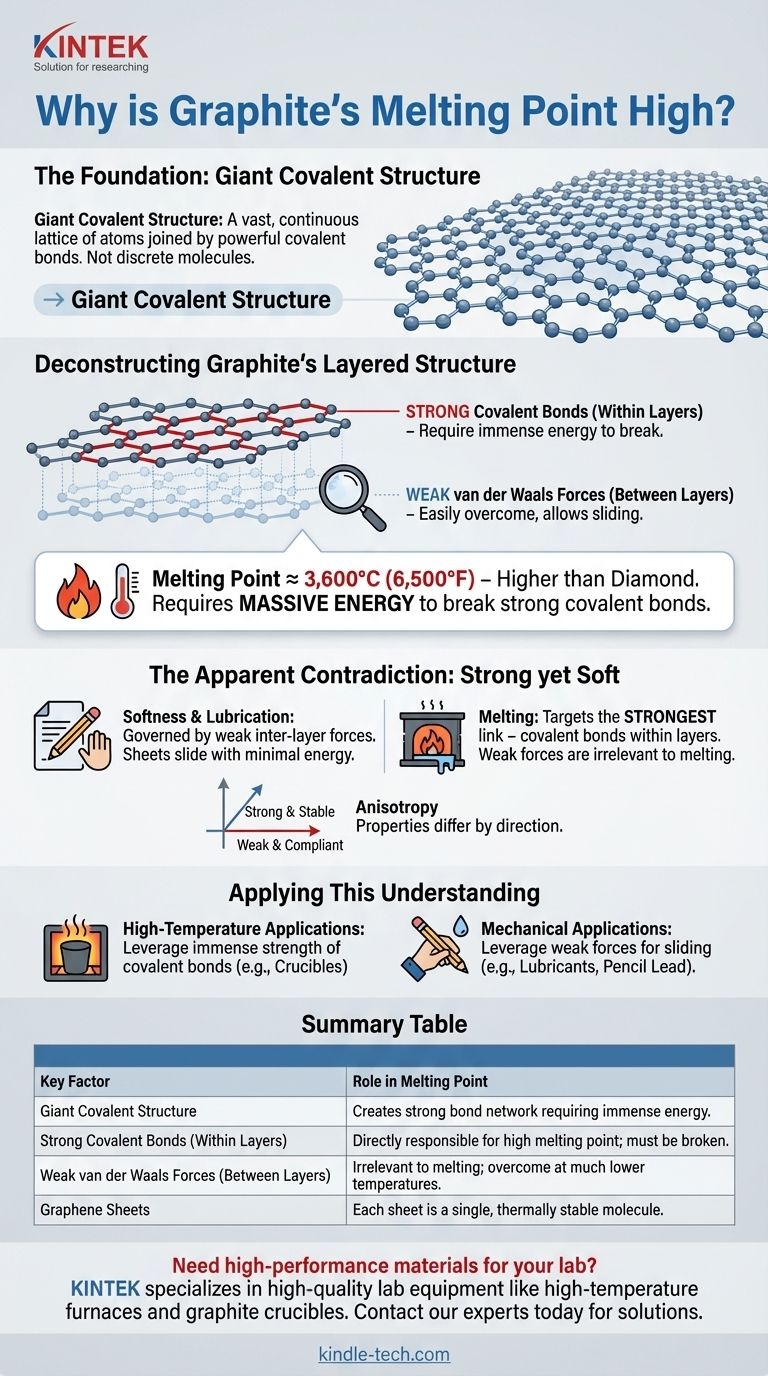
The Foundation: Giant Covalent Structure
A Network of Immense Strength
Graphite is a giant covalent structure, also known as a macromolecular structure. This means it doesn't exist as small, discrete molecules (like water, H₂O) but as a vast, continuous lattice of atoms joined by powerful covalent bonds.
Energy as the Price of Separation
Melting a substance involves giving its atoms enough energy to break free from their fixed positions and move around. In graphite, these positions are locked in by strong covalent bonds.
Breaking these bonds requires a tremendous amount of thermal energy, which translates directly to an extremely high melting point.
Deconstructing Graphite's Layered Structure
The Graphene Sheets: A Covalent Fortress
At the atomic level, graphite is composed of flat, two-dimensional layers. Within each layer, every carbon atom is covalently bonded to three other carbon atoms, forming a hexagonal lattice.
Think of each layer as a single, gigantic molecule—often called a graphene sheet—that is incredibly strong and thermally stable.
The Inter-Layer Gap: Weak van der Waals Forces
While the atoms within a layer are strongly bonded, the forces that hold the different layers together are very weak. These are known as van der Waals forces.
These weak forces are easily overcome, which allows the layers to slide past one another. This is what makes graphite feel soft and slippery, enabling its use in pencils and as a dry lubricant.
The Apparent Contradiction: Strong yet Soft
Misinterpreting Softness
A common point of confusion is how a material can be both soft and have a high melting point. The answer lies in understanding which forces are being overcome for each property.
Softness and lubrication are governed by the weak inter-layer forces. It takes very little energy to make the sheets slide.
Melting Targets the Strongest Link
Melting, however, is not about making layers slide. It's about liberating individual carbon atoms from the powerful covalent bonds within the layers.
Because the covalent bonds are the "strongest link" in the structure, they dictate the thermal stability of the entire material. The weak forces are overcome at much lower temperatures and are not a factor in melting.
Anisotropy: A Directional Material
This dual nature makes graphite highly anisotropic. This means its physical properties are different depending on the direction in which they are measured.
Graphite is strong and thermally stable along the plane of its layers but weak and mechanically compliant perpendicular to them.
Applying This Understanding
Understanding this structural distinction is key to correctly applying graphite in technical and industrial settings.
- If your primary focus is high-temperature applications (like crucibles or furnace linings): Recognize that its high melting point is due to the immense strength of the covalent bonds within its layers, making it exceptionally stable.
- If your primary focus is mechanical applications (like lubricants or pencil lead): Leverage the weak forces between its layers, which allow them to shear and slide easily with minimal force.
- If your primary focus is understanding material science: Appreciate that graphite is a prime example where different bond types within the same material create starkly different properties.
Ultimately, a material's macroscopic properties are a direct and logical consequence of its atomic structure.
Summary Table:
| Key Factor | Role in Melting Point |
|---|---|
| Giant Covalent Structure | Creates a vast network of strong bonds that require immense energy to break. |
| Strong Covalent Bonds (Within Layers) | Directly responsible for the high melting point; these bonds must be broken. |
| Weak van der Waals Forces (Between Layers) | Irrelevant to melting; these forces are overcome at much lower temperatures. |
| Graphene Sheets | Each sheet is a single, thermally stable molecule with high bond strength. |
Need high-performance materials for your lab?
Understanding the properties of materials like graphite is crucial for selecting the right equipment. KINTEK specializes in providing high-quality lab equipment and consumables, including high-temperature furnaces and graphite crucibles that leverage this exceptional thermal stability.
Contact our experts today to find the perfect solutions for your laboratory's specific high-temperature and material science needs.
Visual Guide
Related Products
- Vertical High Temperature Graphite Vacuum Graphitization Furnace
- Ultra-High Temperature Graphite Vacuum Graphitization Furnace
- Graphite Vacuum Furnace High Thermal Conductivity Film Graphitization Furnace
- Graphite Vacuum Continuous Graphitization Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- Why is the graphite furnace technique more sensitive than flame based vaporization methods for atomic absorption? Unlock Superior Trace Analysis
- What is the maximum working temperature of graphite? Unlock High-Temp Performance with the Right Atmosphere
- What is the graphite furnace method? Achieve Ultra-High Temperatures with Purity & Speed
- What is the disadvantage of graphite furnace? Managing Reactivity and Contamination Risks
- What are the steps in the manufacturing of graphite? From Raw Coke to High-Performance Material
- What is the application of graphite furnace? Essential for High-Temp Material Processing & Synthesis
- What is the graphite furnace used for? Achieve Extreme Heat Up to 3000°C in a Controlled Environment
- How does a graphite furnace work? Achieve Extreme Temperatures in a Pure Environment



















