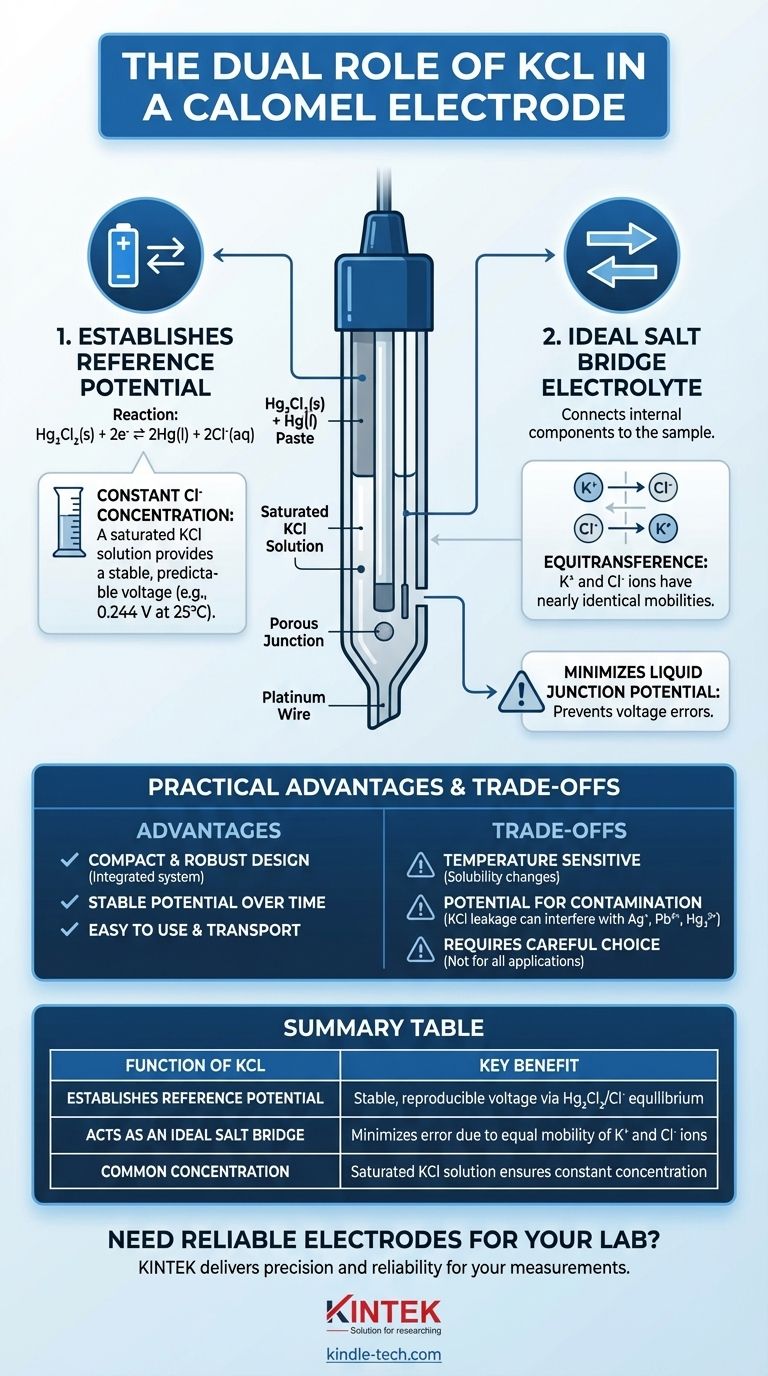
At its core, potassium chloride (KCl) is used in a calomel electrode for two critical reasons. First, the chloride ion (Cl⁻) is a key component in the chemical reaction that establishes the electrode's stable voltage. Second, its potassium (K⁺) and chloride (Cl⁻) ions move at nearly identical speeds in solution, which is the defining characteristic of an ideal salt bridge electrolyte.
The dual function of KCl is the key to the calomel electrode's success. It is not merely a passive component; it actively participates in setting the reference potential while simultaneously preventing measurement errors by functioning as a highly effective salt bridge.

The Dual Role of KCl in Electrode Function
The selection of KCl is a deliberate engineering choice designed to create a stable, reproducible, and convenient reference electrode. It serves two distinct but equally important purposes.
Establishing the Reference Potential
A calomel electrode's voltage is generated by a specific chemical equilibrium: Hg₂Cl₂(s) + 2e⁻ ⇌ 2Hg(l) + 2Cl⁻(aq).
The potential of this reaction is directly dependent on the concentration of chloride ions (Cl⁻) in the solution. By filling the electrode with a KCl solution of a known and constant concentration, a stable and predictable reference potential is established.
Most commonly, a saturated KCl solution is used. This ensures the concentration remains constant even if some water evaporates, providing a highly reproducible voltage that makes it a reliable benchmark for electrochemical measurements.
Functioning as an Ideal Salt Bridge
The KCl solution also acts as a salt bridge, connecting the internal components of the reference electrode to the sample solution you are measuring.
An effective salt bridge requires that its positive and negative ions migrate through the solution at nearly equal rates. This property is known as equitransference.
If one ion moves significantly faster than the other, a charge separation builds up at the boundary between the two solutions. This creates an unwanted voltage known as a liquid junction potential, which introduces a significant error into your measurement.
The mobilities of K⁺ and Cl⁻ ions are almost identical, which minimizes this junction potential and ensures the accuracy of the measurement.
Practical Advantages and Trade-offs
The use of KCl brings significant practical benefits but also introduces considerations that every analyst must understand.
Why KCl is Convenient
The integrated nature of KCl—serving both the electrode reaction and the salt bridge—allows for a compact and robust design.
A calomel electrode does not require a separate, external salt bridge. This makes it easier to set up, use, and transport compared to other reference systems. Its potential is also known to be very stable over time.
The Impact of Concentration
The potential of the calomel electrode is entirely dependent on the KCl concentration. While saturated KCl is most common, other concentrations like 1 M or 0.1 M are also used for specific applications.
Using a saturated solution is convenient because the concentration is self-regulating, but it also means the electrode's potential is more sensitive to changes in temperature, as the solubility of KCl changes with temperature.
Potential for Contamination
A critical trade-off is that the electrode's filling solution can slowly leak into the sample through the porous junction.
If your analysis involves ions that precipitate with chloride, such as silver (Ag⁺), lead (Pb²⁺), or mercury (Hg₂²⁺), the leaking KCl can cause interference and inaccurate results.
Making the Right Choice for Your Goal
Understanding the role of KCl helps you determine when a calomel electrode is the right tool for your specific electrochemical measurement.
- If your primary focus is reproducibility and ease of use: A Saturated Calomel Electrode (SCE) is an excellent and classic choice, provided your sample is free of interfering ions and the temperature is stable.
- If your primary focus is minimizing chloride contamination: You must use a different reference system, such as a mercury-mercurous sulfate electrode, which uses a non-chloride electrolyte.
- If your primary focus is performance across varying temperatures: An electrode with a non-saturated KCl solution (e.g., 3 M) offers a more stable potential with temperature changes than a saturated version.
Ultimately, the choice of KCl is fundamental to the calomel electrode's design, providing the stable foundation required for accurate and reliable measurements.
Summary Table:
| Function of KCl | Key Benefit |
|---|---|
| Establishes Reference Potential | Provides a stable, reproducible voltage via the Hg₂Cl₂/Cl⁻ equilibrium. |
| Acts as an Ideal Salt Bridge | Minimizes measurement error due to nearly equal mobility of K⁺ and Cl⁻ ions. |
| Common Concentration | Saturated KCl solution ensures concentration remains constant, enhancing reproducibility. |
Need a reliable reference electrode for your lab's electrochemical analysis?
At KINTEK, we understand that the accuracy of your measurements depends on high-quality lab equipment. Our range of electrodes and consumables is designed to deliver the precision and reliability your laboratory requires.
Let our experts help you select the perfect equipment for your specific application. Contact KINTEK today to discuss your needs and ensure your lab's success!
Visual Guide
Related Products
- Reference Electrode Calomel Silver Chloride Mercury Sulfate for Laboratory Use
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Metal Disc Electrode Electrochemical Electrode
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Gold Disc Electrode
People Also Ask
- Why is a Glassy Carbon Electrode used as a substrate for paracetamol biomimetic sensors? Expert Substrate Insights
- What is the reference electrode for silver silver chloride? Ag/AgCl is the Standard Itself
- What are the advantages of BDD electrodes? Maximize Wastewater Treatment Efficiency and Durability
- What are the functions of a glassy carbon electrode in CV testing of antioxidants? Enhance Your Redox Analysis Accuracy
- What are the advantages of graphite material? Superior Thermal & Electrical Performance for Extreme Conditions
- How is a polished electrode tested for quality? Validate Performance with Cyclic Voltammetry
- What precautions should be taken when connecting a platinum disk electrode? Ensure Accurate Measurements & Longevity
- Why are non-active BDD anodes selected for wastewater treatment? Achieve Total Pollutant Mineralization



















