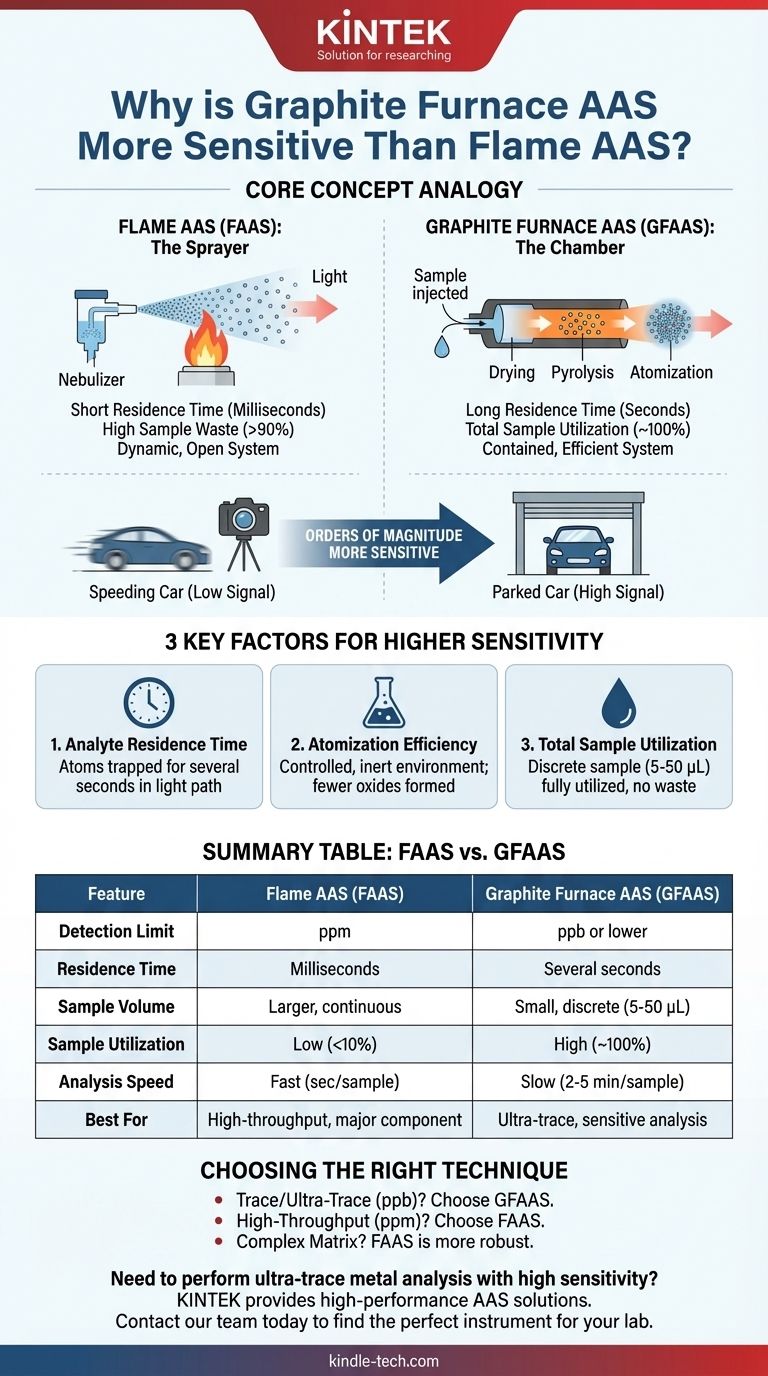
In analytical chemistry, the graphite furnace technique is orders of magnitude more sensitive than flame-based atomic absorption because it excels at two fundamental things: efficiently converting the sample into free atoms and confining those atoms within the instrument's light path for a much longer time. This extended residence time allows for a stronger, more detectable absorption signal from a very small amount of sample.
The core difference is one of containment versus dispersion. A flame atomizer rapidly disperses a continuous spray of sample, wasting most of it and allowing atoms to pass through the light beam in milliseconds. A graphite furnace atomizes a discrete sample in a contained tube, creating a dense cloud of atoms that remains in the light path for several seconds, dramatically increasing the measured signal.

The Role of the Atomizer: Flame vs. Furnace
Atomic absorption spectroscopy (AAS) relies on converting an element into a cloud of free, ground-state atoms that can absorb light. The device that accomplishes this is called the atomizer, and its design is the primary determinant of the instrument's sensitivity.
The Flame Atomizer (FAAS): A Dynamic, Open System
In Flame AAS, the liquid sample is continuously aspirated into a nebulizer, which creates a fine aerosol. This aerosol is mixed with fuel and oxidant gases and carried into a flame.
The flame's heat desolvates the sample and breaks down chemical compounds to produce free atoms. However, this process is very inefficient. The high gas flow rate means that atoms only spend a few milliseconds in the light path before being swept out of the flame.
Furthermore, a large portion of the initial sample (often over 90%) is simply drained away and never reaches the flame.
The Graphite Furnace Atomizer (GFAAS): A Contained, Efficient System
In Graphite Furnace AAS, a small, discrete volume of sample (typically 5-50 microliters) is injected into a graphite tube. This tube is then heated in a pre-programmed, multi-step sequence.
First, a low-temperature drying step evaporates the solvent. Next, a higher-temperature pyrolysis (or charring) step removes volatile matrix components. Finally, the tube is rapidly heated to a very high temperature (up to 3000 °C) for atomization, instantly creating a dense cloud of atoms inside the confined space of the tube.
Unpacking the Sources of Higher Sensitivity
The architectural difference between the two atomizers directly leads to the superior sensitivity of GFAAS. This can be attributed to three key factors.
Key Factor 1: Analyte Residence Time
This is the most significant factor. In FAAS, atoms rush through the light path in milliseconds. In GFAAS, the graphite tube physically traps the atom cloud, resulting in a residence time of several seconds.
Think of it like trying to photograph a car. FAAS is like trying to get a clear picture of a car speeding down the highway, while GFAAS is like taking a picture of the same car parked in a garage. The longer observation time allows the detector to measure a much more significant and integrated absorption signal.
Key Factor 2: Atomization Efficiency
The controlled, oxygen-free (inert argon gas) environment of the graphite furnace is more efficient at producing free atoms than a flame. The programmed heating removes much of the sample matrix before the final, high-temperature atomization step.
A hot, turbulent flame is an aggressive and complex chemical environment. It can easily form stable metal oxides that do not absorb light at the desired wavelength, reducing the population of free atoms and thus lowering the signal.
Key Factor 3: Total Sample Utilization
GFAAS atomizes virtually 100% of the discrete sample that is injected into the tube. This creates a very high concentration of atoms within the small, fixed volume of the furnace.
FAAS, by contrast, is a high-waste technique. The continuous aspiration process requires a much larger sample volume, but most of it is discarded by the nebulizer system, and the atoms that are created are diluted in a large flame volume.
Understanding the Trade-offs
While GFAAS offers superior sensitivity, this performance comes with significant trade-offs. It is not always the better choice.
Speed and Sample Throughput
FAAS is fast. A typical measurement takes only a few seconds per sample, making it ideal for high-throughput labs analyzing many samples.
GFAAS is slow. Each analysis requires the full heating and cooling cycle of the graphite tube, which can take 2 to 5 minutes per sample.
Precision and Interferences
Because FAAS measures a steady-state signal over several seconds, it generally offers better precision (reproducibility) than the transient, peak-shaped signal from GFAAS.
GFAAS is also far more susceptible to matrix interferences and background absorption from smoke and molecular species generated during atomization. This necessitates more advanced and effective background correction systems (e.g., Zeeman or Deuterium arc) to achieve accurate results.
Cost and Complexity
Graphite furnace systems are significantly more expensive to purchase and operate than flame systems. The graphite tubes are consumable items that must be replaced regularly.
Method development for GFAAS is also more complex, requiring careful optimization of the multi-step temperature program for each different sample type.
Choosing the Right Technique for Your Analysis
The choice between Flame and Graphite Furnace AAS is a classic analytical decision based on balancing the need for sensitivity against practical considerations like speed, cost, and robustness.
- If your primary focus is trace or ultra-trace analysis (ppb or lower): GFAAS is the only viable choice. Its superior sensitivity is required to detect elements at these low concentrations.
- If your primary focus is high-throughput or routine analysis of major components (ppm level): FAAS is far more practical. Its speed, lower cost, and simplicity make it the workhorse for quality control and routine monitoring.
- If your samples have a very complex or unknown matrix: FAAS is often a more robust starting point. It is less prone to the severe physical and chemical interferences that can plague GFAAS analysis.
Ultimately, understanding these fundamental principles allows you to select the instrument not just for its performance specifications, but for its suitability to your specific analytical challenge.
Summary Table:
| Feature | Flame AAS (FAAS) | Graphite Furnace AAS (GFAAS) |
|---|---|---|
| Detection Limit | Parts-per-million (ppm) | Parts-per-billion (ppb) or lower |
| Analyte Residence Time | Milliseconds | Several seconds |
| Sample Volume | Larger volume, continuous aspiration | Small, discrete volume (5-50 µL) |
| Sample Utilization | Low (<10%) | High (~100%) |
| Analysis Speed | Fast (seconds/sample) | Slow (2-5 minutes/sample) |
| Best For | High-throughput, major component analysis | Ultra-trace, sensitive analysis |
Need to perform ultra-trace metal analysis with high sensitivity?
KINTEK specializes in providing high-performance laboratory equipment, including advanced atomic absorption spectrometers. Our experts can help you select the right instrument—whether it's a robust flame system for high-throughput work or a sensitive graphite furnace for trace analysis—to meet your specific laboratory needs and ensure accurate, reliable results.
Contact our team today to discuss your application and find the perfect AAS solution for your lab.
Visual Guide
Related Products
- Graphite Vacuum Continuous Graphitization Furnace
- Vacuum Hot Press Furnace Machine for Lamination and Heating
- Metallographic Specimen Mounting Machine for Laboratory Materials and Analysis
- Laboratory Sterilizer Lab Autoclave Pulsating Vacuum Desktop Steam Sterilizer
- Desktop Fast High Pressure Laboratory Autoclave Sterilizer 16L 24L for Lab Use
People Also Ask
- What happens to graphite at high temperatures? Unlock its Extreme Heat Resistance
- How is synthetic graphite manufactured? A Deep Dive into the High-Temperature Process
- What is the thermal limit of graphite? Unlock Extreme Heat Performance in Your Lab
- Why can graphite withstand heat? Unlocking Its Extreme Thermal Stability for Your Lab
- Is a graphite melting point high or low? Discover Its Extreme Thermal Resilience

















