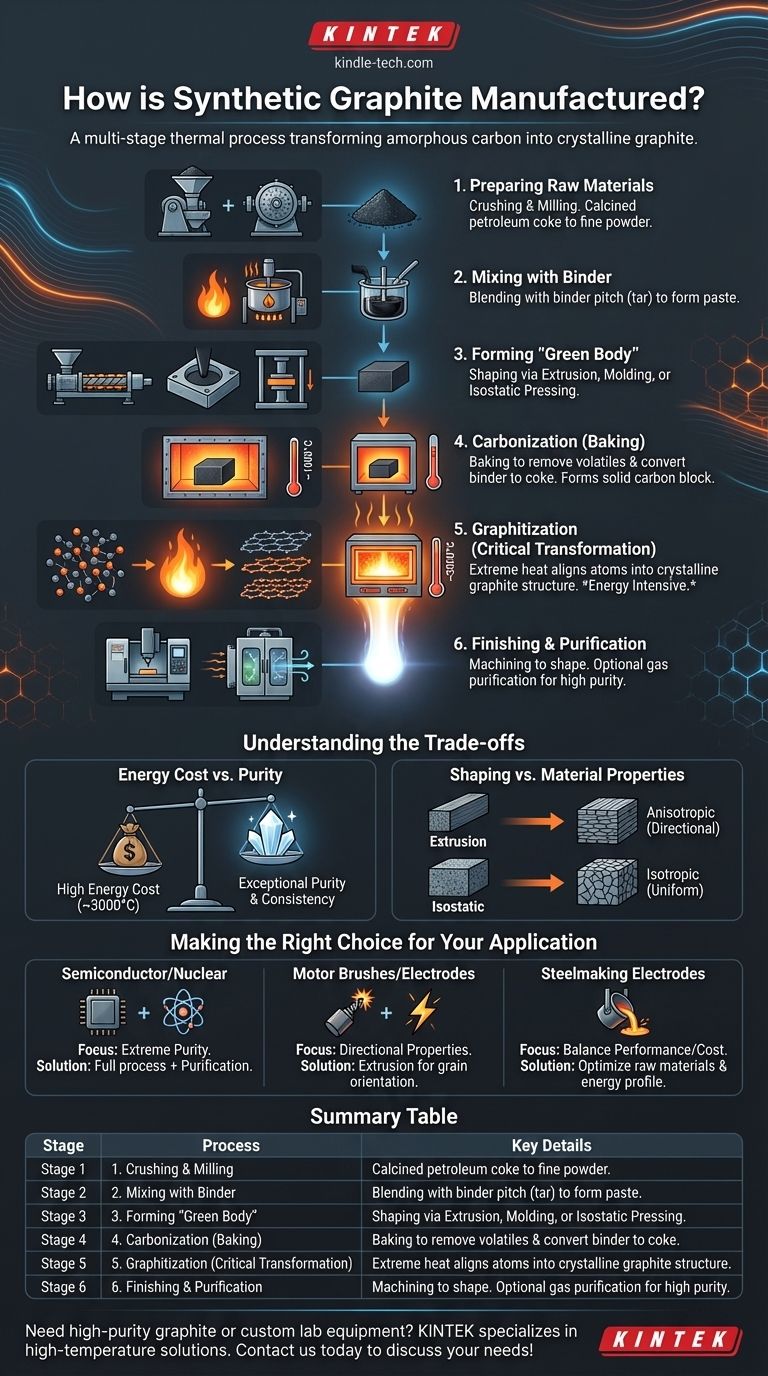
In essence, manufacturing synthetic graphite is a highly controlled, multi-stage thermal process designed to transform amorphous carbon precursors into a pure, crystalline graphite structure. It begins by mixing carbonaceous raw materials like petroleum coke with a binder, shaping this mixture into a desired form, and then subjecting it to two critical high-temperature heating cycles—carbonization around 1000°C and graphitization near 3000°C.
The core principle of synthetic graphite production is not one of simple melting and casting, but of solid-state transformation. It is an energy-intensive journey that forces disordered carbon atoms to rearrange themselves into the highly ordered, layered structure that gives graphite its unique electrical and thermal properties.

The Manufacturing Blueprint: From Raw Carbon to Engineered Graphite
The production of synthetic graphite is a precise sequence of steps, each engineered to control the final properties of the material. The journey starts with simple carbon powders and ends with a high-performance engineered product.
Stage 1: Preparing the Raw Materials
The process begins with solid carbonaceous raw materials, most commonly calcined petroleum coke and sometimes existing graphite powders. These materials are first crushed and milled into a fine, controlled particle size distribution.
This initial step is critical because the size and mix of these particles directly influence the density, mechanical strength, and uniformity of the final graphite product.
Stage 2: Mixing with a Binder
The milled carbon powder is then heated and blended with a binder pitch, a thick, tar-like substance derived from coal tar or petroleum. The mixture is combined in a heated mixer until a homogeneous, paste-like mass is formed.
The binder acts as a "glue," coating the carbon particles and providing the plasticity needed for the mixture to be shaped in the next stage.
Stage 3: Forming the "Green Body"
The warm, pliable carbon-binder mixture is then shaped into what is known as a "green body" (an unbaked, raw form). The shaping method used depends on the desired final geometry and properties.
Common methods include:
- Extrusion: Pushing the paste through a die to form long rods or tubes.
- Vibration Molding: Compacting the material into large rectangular or cylindrical molds.
- Isostatic Pressing: Applying high pressure from all directions to the material in a flexible mold, resulting in a highly uniform and dense product.
Stage 4: Carbonization (Baking)
The green body is then carefully loaded into a furnace for carbonization, also known as baking. It is slowly heated in an oxygen-free environment to approximately 1000°C.
This crucial step bakes out volatile compounds from the binder pitch and converts the binder into solid carbon, known as "coke." This process rigidly locks the original carbon particles together, creating a hard, brittle, and electrically conductive block of amorphous carbon.
Stage 5: Graphitization (The Critical Transformation)
The baked carbon block is then subjected to the defining step: graphitization. The material is heated in an electric furnace to extremely high temperatures, typically between 2800°C and 3000°C.
This immense thermal energy forces the disordered carbon atoms from the coke and binder to rearrange into the orderly, hexagonal, layered crystalline structure of graphite. This is where the material acquires its signature properties: high electrical conductivity, excellent thermal conductivity, and lubricity.
Stage 6: Finishing and Purification
After cooling, the synthetic graphite block can be machined into precise final shapes for specific applications, like electrodes or heating elements.
For high-purity applications (e.g., semiconductors, nuclear reactors), the graphite may undergo an additional gas-based purification process at high temperatures to remove the last traces of mineral impurities.
Understanding the Trade-offs
The choice to use synthetic graphite and the specifics of its manufacturing process are governed by a clear set of trade-offs between cost, performance, and final properties.
Energy Cost vs. Purity and Performance
The graphitization step, requiring temperatures near 3000°C, is incredibly energy-intensive and expensive. This is the primary driver of synthetic graphite's higher cost compared to most natural graphite.
However, this cost is justified by the exceptional purity and highly consistent, predictable properties that can be achieved. Unlike natural graphite, which contains variable mineral impurities, synthetic graphite can be manufactured to meet exacting specifications.
Shaping Method vs. Material Properties
The forming method used in Stage 3 has a direct impact on the material's internal structure.
Extrusion tends to align the graphite crystals parallel to the direction of extrusion, creating an anisotropic material with different properties (e.g., conductivity) when measured in different directions. In contrast, isostatic pressing produces a more random crystal orientation, resulting in an isotropic material with uniform properties in all directions.
Making the Right Choice for Your Application
The manufacturing process can be tailored to achieve specific outcomes, making it critical to align the process with the end goal.
- If your primary focus is extreme purity and predictable performance (e.g., semiconductor or nuclear applications): The full, multi-stage process including a final, high-temperature gas purification step is essential to remove all impurities.
- If your primary focus is creating specific shapes with directional properties (e.g., electrical motor brushes or EDM electrodes): The choice of shaping method, particularly extrusion, becomes the most critical decision to control the grain orientation.
- If your primary focus is balancing performance with cost (e.g., steelmaking electrodes): The key is optimizing the selection of raw coke materials and the energy profile of the graphitization cycle to meet performance targets without excessive cost.
Understanding this manufacturing pathway empowers you to see synthetic graphite not as a raw material, but as an engineered solution crafted for a purpose.
Summary Table:
| Stage | Process | Key Details |
|---|---|---|
| 1 | Raw Material Prep | Crushing and milling of calcined petroleum coke |
| 2 | Mixing with Binder | Blending with coal tar or petroleum pitch |
| 3 | Forming | Extrusion, molding, or isostatic pressing |
| 4 | Carbonization | Baking at ~1000°C to remove volatiles |
| 5 | Graphitization | Heating to 2800-3000°C for crystal alignment |
| 6 | Finishing | Machining and optional purification |
Need high-purity graphite or custom lab equipment? KINTEK specializes in lab equipment and consumables, including materials for high-temperature processing. Our expertise ensures you get the right solutions for semiconductor, nuclear, or industrial applications. Contact us today to discuss your specific needs and benefit from our engineered materials and support!
Visual Guide
Related Products
- Vertical High Temperature Graphite Vacuum Graphitization Furnace
- Graphite Vacuum Continuous Graphitization Furnace
- Ultra-High Temperature Graphite Vacuum Graphitization Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- At what temperature does graphite melt? Understanding Its Extreme Phase Change
- Why is graphite the best conductor of heat? Understanding Its Directional Thermal Superiority
- What is the graphite furnace used for? Achieve Extreme Heat Up to 3000°C in a Controlled Environment
- Is graphite affected by heat? Discover Its Remarkable Strength and Stability at High Temperatures
- What temperature can graphite handle? Unlocking its extreme heat resistance in inert environments



















