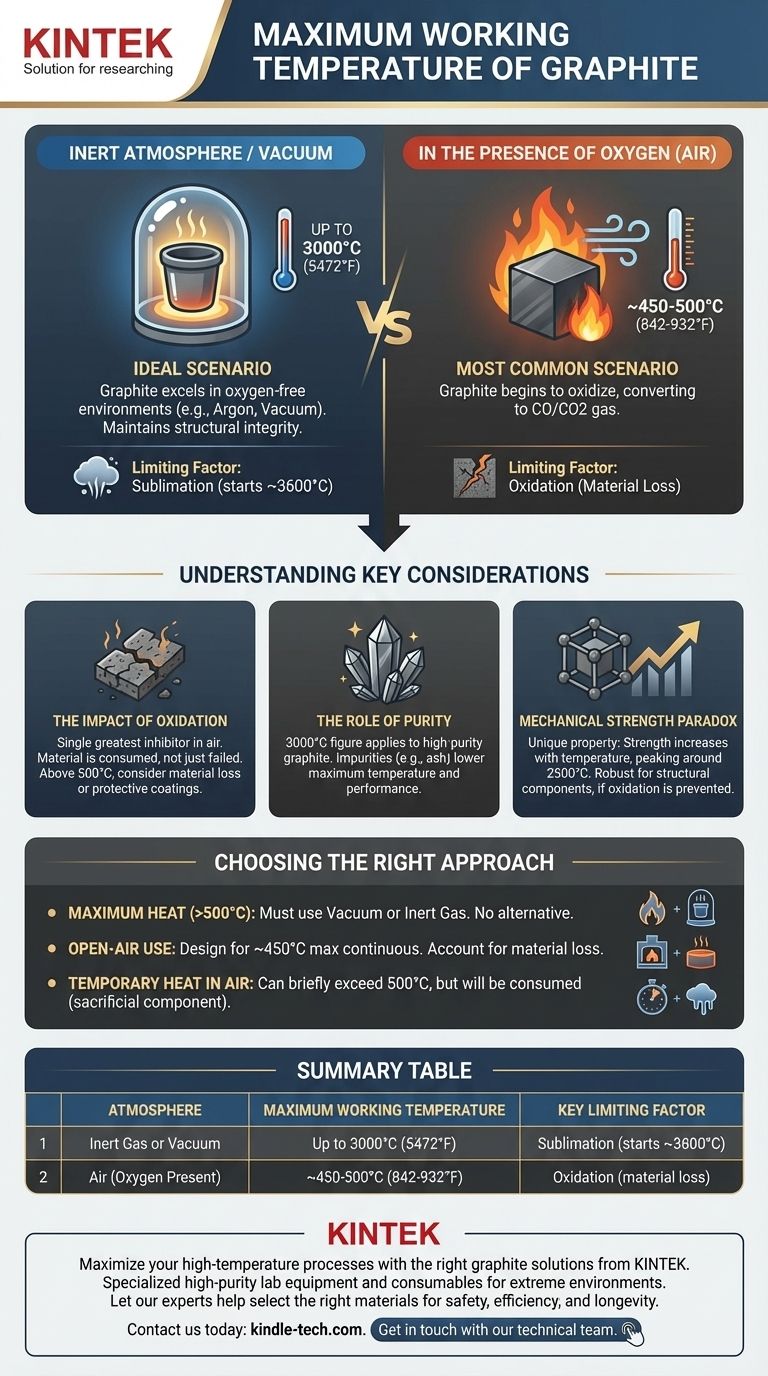
Under ideal conditions, the maximum working temperature of high-purity graphite is exceptionally high, reaching up to 3000°C (5472°F). This makes it a premier material for high-temperature applications like crucibles for melting metals. However, this figure is only achievable in a specific type of environment.
The true maximum temperature of graphite is not a fixed number. It is critically dependent on the surrounding atmosphere—specifically, the presence or absence of oxygen.

The Decisive Factor: Atmosphere
Graphite's performance at high temperatures is a tale of two drastically different environments. The theoretical maximum is only relevant when you control the atmosphere around it.
In an Inert Atmosphere or Vacuum
This is the ideal scenario where graphite truly excels.
In an environment free of oxygen, such as a vacuum or a furnace filled with an inert gas like argon, graphite maintains its structural integrity to extreme temperatures.
Here, the primary limitation is sublimation, where the solid carbon turns directly into a gas. This process begins around 3600°C, so a working temperature of 3000°C provides a safe and effective operational ceiling.
In the Presence of Oxygen (Air)
This is the most common real-world scenario and the most significant limitation.
When heated in the presence of oxygen, graphite begins to oxidize. This is a chemical reaction that converts the solid graphite into CO and CO2 gas, effectively consuming the material.
This oxidation process starts to become significant at temperatures as low as 450-500°C (842-932°F). Above this, the rate of material loss increases rapidly, severely limiting the component's lifespan and effectiveness.
Understanding the Key Considerations
To properly apply graphite, you must understand the trade-offs between its potential and its practical limitations. The difference between success and failure often lies in controlling for oxidation.
The Impact of Oxidation
Oxidation is the single greatest inhibitor to using graphite at high temperatures in air.
It's not a failure of the material melting or cracking; it's a process of it being eaten away. For any long-term application in air above 500°C, you must consider the rate of material loss or use a protective coating.
The Role of Purity
The 3000°C figure applies to high-purity graphite.
Impurities, such as ash content, can lower the material's maximum temperature and negatively affect its performance. For demanding applications like semiconductor manufacturing or laboratory crucibles, higher purity is essential.
Mechanical Strength Paradox
Unlike metals that soften when heated, graphite's mechanical strength actually increases with temperature, peaking around 2500°C.
This unique property makes it incredibly robust for high-temperature structural components, provided oxidation is prevented.
Choosing the Right Approach for Your Application
Your intended use case directly dictates the relevant temperature limit you must respect.
- If your primary focus is achieving maximum heat (above 500°C): You must operate within a vacuum or an inert gas atmosphere. There is no alternative.
- If your primary focus is use in an open-air environment: You must design around a much lower maximum continuous temperature of approximately 450°C and account for material loss over time.
- If you need a temporary heat source in air: Graphite can briefly exceed 500°C, but it will be consumed in the process, making it a sacrificial component.
By understanding that atmosphere, not temperature alone, is graphite's true limiting factor, you can properly engineer its remarkable capabilities into your project.
Summary Table:
| Atmosphere | Maximum Working Temperature | Key Limiting Factor |
|---|---|---|
| Inert Gas or Vacuum | Up to 3000°C (5472°F) | Sublimation (starts ~3600°C) |
| Air (Oxygen Present) | ~450-500°C (842-932°F) | Oxidation (material loss) |
Maximize your high-temperature processes with the right graphite solutions from KINTEK.
Whether you're melting metals in crucibles or conducting high-heat experiments, the performance of your graphite components depends critically on the operating atmosphere. KINTEK specializes in high-purity lab equipment and consumables, including graphite designed for extreme environments.
Let our experts help you select the right materials and configurations to ensure safety, efficiency, and longevity in your applications. Contact us today to discuss your specific needs and discover how our solutions can enhance your laboratory's capabilities.
Get in touch with our technical team
Visual Guide
Related Products
- Graphite Vacuum Continuous Graphitization Furnace
- Vertical High Temperature Graphite Vacuum Graphitization Furnace
- Ultra-High Temperature Graphite Vacuum Graphitization Furnace
- Graphite Vacuum Furnace High Thermal Conductivity Film Graphitization Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- Why is a graphite furnace more sensitive than a flame? Unlocking Superior Trace Analysis
- What are the stages of graphite furnace? A Guide to Precise Multi-Stage Temperature Programming
- Why is a graphite furnace more sensitive than a flame atomizer? Unlock the Physics of Trace-Level Analysis
- Why is a graphite furnace rather than a flame often used for atomization? Superior Sensitivity for Trace Analysis
- What is the purpose of carbonization? Transform Organic Materials into Valuable Carbon Products
- What are the disadvantages of graphite furnace? Key Limitations and Operational Costs
- What is the temperature range of a graphite furnace? Unlock up to 3000°C for advanced materials processing.
- How does a graphite furnace work? Achieve Extreme Temperatures in a Pure Environment



















