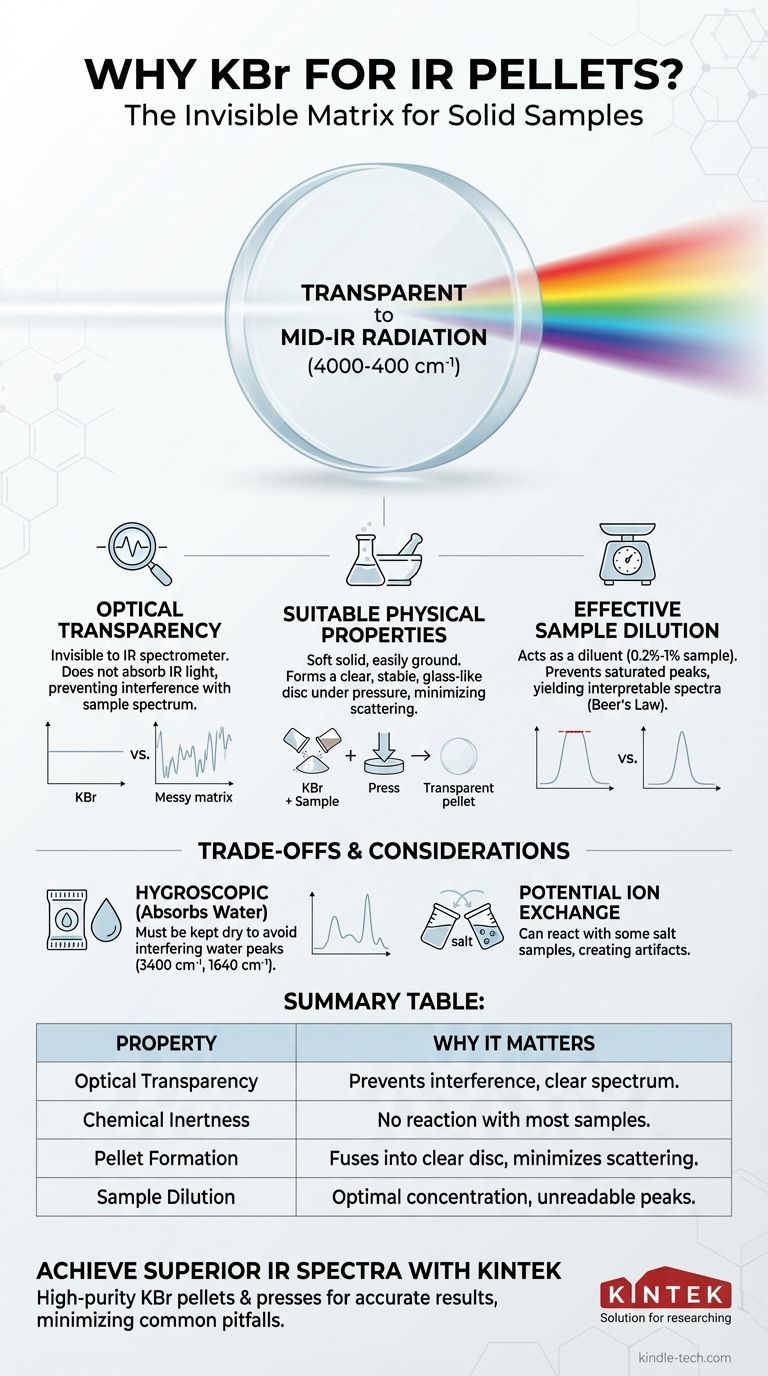
Potassium Bromide (KBr) is used for preparing solid samples for infrared (IR) spectroscopy because it is transparent to IR radiation across a wide spectral range and possesses the physical properties needed to form a stable, clear pellet under pressure. This allows the sample to be analyzed in a solid state without interference from the surrounding matrix.
KBr's primary value is its "invisibility" to the IR spectrometer, acting as a neutral matrix that holds the sample without interfering with its spectral measurement. This optical transparency, combined with its ability to form a solid, glass-like disc, makes it the default choice for analyzing solid compounds.

The Core Requirements of a Sample Matrix
To analyze a solid sample using transmission IR spectroscopy, the sample must be held within a medium, or matrix, that is transparent to the IR beam. An ideal matrix must satisfy several key requirements.
Optical Transparency
The most critical property is that the matrix material itself must not absorb infrared radiation in the region of interest. If the matrix absorbed the light, its own spectrum would overlap with and obscure the spectrum of the sample being analyzed.
Chemical Inertness
The matrix must be chemically inert and not react with the sample. Any reaction would create new chemical species, resulting in a spectrum that does not represent the original compound.
Suitable Physical Properties
The material must be a soft solid that can be finely ground into a powder. Critically, when subjected to high pressure, this powder must be able to deform and fuse into a homogeneous, transparent, or translucent disc that allows the IR beam to pass through with minimal scattering.
Why KBr Excels as a Pellet Material
Potassium Bromide meets all the core requirements for an IR matrix, making it the industry standard for creating pellets from solid samples.
An Exceptionally Wide Transparent Window
KBr is transparent across the entire mid-infrared range, from 4000 cm⁻¹ down to approximately 400 cm⁻¹. This covers the vast majority of vibrational frequencies for organic and inorganic functional groups, ensuring the KBr itself does not produce interfering peaks.
Formation of a Solid "Glass"
KBr is a crystalline salt that is relatively soft. When ground into a fine powder and mixed with the sample, this mixture can be pressed in a die at high pressure (several tons). The pressure causes the soft KBr crystals to deform and fuse, a process called plastic flow.
This process creates a solid, glass-like pellet that effectively traps the finely dispersed sample molecules within the KBr lattice. This uniformity minimizes the scattering of the IR beam, which would otherwise lead to a noisy and uninterpretable spectrum.
Proper Sample Dilution
As a matrix, KBr also serves as a diluent. Solid samples are often too concentrated to analyze directly. A thick, pure sample would absorb 100% of the IR light at its characteristic frequencies, leading to "flat-topped" peaks and loss of quantitative information.
By mixing a small amount of sample (typically 0.2% to 1%) into the KBr, a pellet of manageable thickness can be created where the absorption follows Beer's Law, yielding a high-quality, interpretable spectrum.
Understanding the Trade-offs and Common Pitfalls
While KBr is the standard, it is not without its challenges. Understanding these limitations is key to acquiring a clean spectrum.
The Problem of Water Absorption
KBr is hygroscopic, meaning it readily absorbs moisture from the atmosphere. This is its single biggest drawback. Water has strong, distinct IR absorption bands: a very broad peak around 3400 cm⁻¹ (O-H stretch) and a sharp peak around 1640 cm⁻¹ (H-O-H bend).
If your KBr is "wet," these water peaks will appear in your spectrum and can obscure important sample peaks. For this reason, spectroscopic-grade KBr must be kept in a desiccator or dried in an oven before use.
Potential for Ion Exchange
Because KBr is an ionic salt (K⁺Br⁻), it can sometimes react with samples that are also salts, particularly hydrochloride salts of amines (R-NH₃⁺Cl⁻). In these cases, a halide exchange can occur, where some of the sample's chloride is replaced by bromide from the matrix. This creates a new compound and an artifact in the spectrum.
When Other Materials are Needed
For analysis in the far-infrared region (below 400 cm⁻¹), KBr begins to absorb light. In these cases, a different matrix like Cesium Iodide (CsI), which is transparent down to 200 cm⁻¹, must be used. For samples that are highly water-sensitive or reactive, non-pellet methods like a Nujol mull or Attenuated Total Reflectance (ATR) are better alternatives.
Making the Right Choice for Your Sample
Your choice of sample preparation method depends entirely on your analytical goal and the nature of your compound.
- If your primary focus is routine analysis of stable organic compounds: KBr is the cost-effective and reliable standard, provided you take precautions to control for moisture.
- If your analysis extends into the far-infrared region (below 400 cm⁻¹): You must switch to a different matrix like Cesium Iodide (CsI) to avoid matrix absorption.
- If your sample is aqueous, reactive with halide ions, or difficult to grind: Consider alternative sampling methods like Attenuated Total Reflectance (ATR) spectroscopy, which requires minimal to no sample preparation.
Ultimately, understanding the properties of your matrix is as crucial as understanding your sample itself for achieving a clean and accurate spectrum.
Summary Table:
| Property | Why It Matters for IR Pellets |
|---|---|
| Optical Transparency | KBr does not absorb IR light in the key mid-IR range (4000-400 cm⁻¹), preventing interference. |
| Chemical Inertness | It does not react with most samples, ensuring the spectrum represents the original compound. |
| Pellet Formation | Under pressure, KBr fuses into a clear, solid disc that minimizes light scattering. |
| Sample Dilution | It allows for optimal sample concentration to avoid saturated, unreadable peaks. |
Ready to achieve superior results in your lab?
KINTEK specializes in high-purity laboratory equipment and consumables, including spectroscopic-grade KBr pellets and presses. Our products are designed to help you obtain the clear, accurate IR spectra your research demands, while minimizing common pitfalls like moisture absorption.
Contact us today to discuss your specific laboratory needs and let our experts help you select the right tools for your success.
Get in touch with our team now!
Visual Guide
Related Products
- Laboratory Hydraulic Press Split Electric Lab Pellet Press
- kbr pellet press 2t
- Automatic Laboratory Hydraulic Press for XRF & KBR Pellet Press
- Laboratory Manual Hydraulic Pellet Press for Lab Use
- Laboratory Hydraulic Press Lab Pellet Press Machine for Glove Box
People Also Ask
- What role does a benchtop incubator shaker play during antimicrobial activity assessment? Ensure Precise Results
- What are the guidelines for sintering design? A Systematic Approach to Material Density and Strength
- What is the difference between electric arc furnace and plasma arc furnace? Choose the Right Tool for Your Heat Processing Needs
- What is the difference between sintering and powder metallurgy? Sintering is a Key Step Within the Process
- What are the purposes of brazing? Achieve Strong, Leak-Proof Joints with Minimal Heat Stress
- Why is vacuum used in evaporator? Unlock Efficient, Low-Temperature Evaporation
- What are the advantages of reactive sputtering? Achieve Precise Control Over Compound Thin Films
- What are the fundamentals of sputtering? Master the Art of High-Quality Thin Film Deposition



















