The Struggle Against Entropy
Science is, effectively, the art of controlling variables in a chaotic world.
Among these variables, temperature is the most notoriously difficult to tame. It wants to fluctuate. It wants to escape. It wants to be uneven.
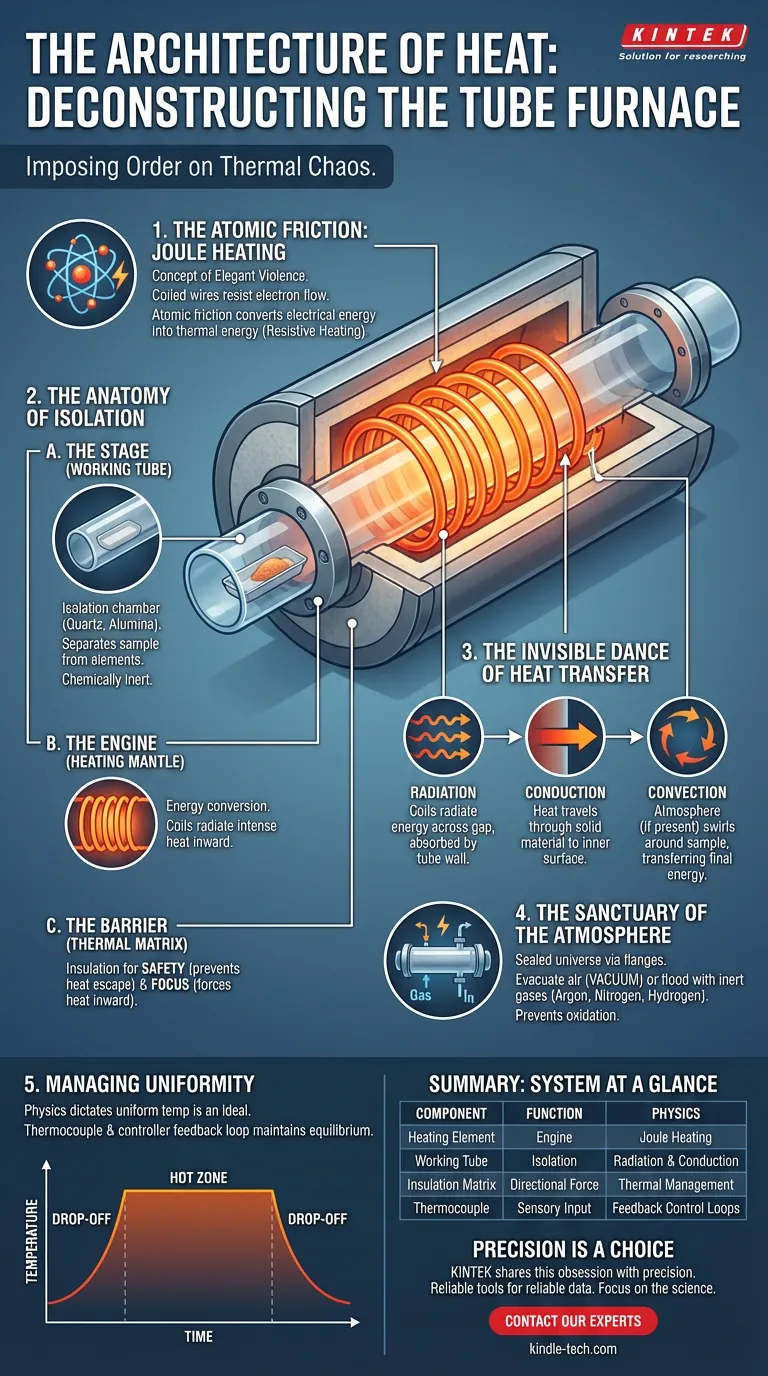
The tube furnace is not merely a heater. It is a carefully engineered fortress designed to impose order on thermal chaos. At its most fundamental level, it turns raw electricity into a distinct, repeatable environment where discovery can happen.
To understand the equipment is to understand the difference between a failed experiment and a material breakthrough.
The Atomic Friction: How It Begins
The principle driving a tube furnace is Joule heating, also known as resistive heating. It is a concept of elegant violence.
Inside the furnace’s "mantle," coiled wires surround the central chamber. When we force an electric current through these coils, the material resists the flow of electrons.
This resistance creates friction at an atomic level. The electrical energy, finding nowhere else to go, converts into thermal energy.
It is the same principle inside your kitchen toaster, but engineered with a level of precision that allows for the synthesis of advanced nanomaterials rather than just browning bread.
The Anatomy of Isolation
A tube furnace is a system of three distinct layers, each serving a specific role in the management of energy.
1. The Stage (The Working Tube)
The heart of the system is a long, cylindrical vessel. This is the working tube.
Made from quartz, alumina, or specialized alloys, it serves as the isolation chamber. It physically separates your sample from the heating elements. Its job is to survive the thermal shock while remaining chemically inert.
2. The Engine (The Heating Mantle)
Surrounding the tube is the heating mantle. This is where the energy conversion happens. The coils here glow with radiant intensity, projecting heat inward.
3. The Barrier (The Thermal Matrix)
The heating coils are embedded in a thermally insulating matrix. This insulation performs a dual function:
- Safety: It prevents heat from escaping outward.
- Focus: It forces the thermal energy to travel in the only direction available—inward, toward the sample.
The Invisible Dance of Heat Transfer
Most people assume a furnace heats things by touching them. In a tube furnace, the process is more sophisticated.
It begins with Radiation. The heating coils do not touch the tube. They radiate energy across the gap. This thermal radiation is absorbed by the outer wall of the working tube.
It moves to Conduction. The heat travels through the solid material of the tube wall, reaching the inner surface.
It finishes with Convection. If there is gas inside the tube, that atmosphere heats up and swirls around your sample boat, transferring the final joules of energy needed for the reaction.
The Sanctuary of the Atmosphere
The true genius of the tube furnace design lies in what it keeps out.
By fitting flanges to the ends of the tube, you create a sealed universe. You can evacuate the air to create a vacuum, or flood the chamber with argon, nitrogen, or hydrogen.
This allows you to process materials that would otherwise oxidize and ruin in a standard open-air box furnace. It creates a sanctuary where the only chemistry occurring is the chemistry you invited.
The Illusion of Uniformity (And How to Manage It)
There is a psychological gap in laboratory work: we tend to trust the digital number on the controller display implicitly.
However, physics dictates that a "uniform temperature" is an ideal, not a default.
- The Hot Zone: Heat is most stable in the geometric center of the tube.
- The Drop-off: Near the ends of the tube, where insulation is thinner or flanges act as heat sinks, the temperature drops.
- The Feedback Loop: A thermocouple constantly senses the temperature and talks to the controller. This is a negotiation. The controller pulses power to the coils to maintain equilibrium.
Understanding these behaviors allows you to place your sample exactly where the physics is most favorable.
Summary: The System at a Glance
| Component | The Function | The Physics |
|---|---|---|
| Heating Element | The engine of the system | Joule (Resistive) Heating |
| Working Tube | The isolation chamber | Thermal Radiation & Conduction |
| Insulation Matrix | The directional force | Thermal Management |
| Thermocouple | The brain's sensory input | Feedback Control Loops |
Precision is a Choice
The tube furnace is a testament to the engineer’s desire for predictability. It is a machine built to ensure that when you ask for 1,200°C in an argon atmosphere, you get exactly that—no more, no less.
At KINTEK, we share this obsession with precision.
Our tube furnaces are designed for researchers who understand that the quality of the equipment dictates the reliability of the data. From superior temperature uniformity to robust atmospheric controls, we build the tools that allow you to focus on the science, not the troubleshooting.
Contact Our Experts to discuss your specific thermal processing needs.
Visual Guide
Related Products
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
- Multi Heating Zones CVD Tube Furnace Machine Chemical Vapor Deposition Chamber System Equipment
Related Articles
- Your Tube Furnace Is Not the Problem—Your Choice of It Is
- The Anatomy of Control: Why Every Component in a Tube Furnace Matters
- Mastering the Micro-Environment: Why the Tube Furnace Is a Scientist's Most Powerful Tool for Innovation
- Cracked Tubes, Contaminated Samples? Your Furnace Tube Is The Hidden Culprit
- High Pressure Tube Furnace: Applications, Safety, and Maintenance




















