Yes, you can absolutely braze stainless steel, but it requires a specific approach. Unlike brazing simpler metals, the process must overcome the material's naturally protective and highly stable oxide layer to achieve a strong, reliable joint.
The central challenge in brazing stainless steel is not the steel itself, but the invisible, resilient layer of chromium oxide on its surface. The success of the entire process hinges on effectively removing this layer and preventing it from reforming, with vacuum brazing being the premier industrial solution.

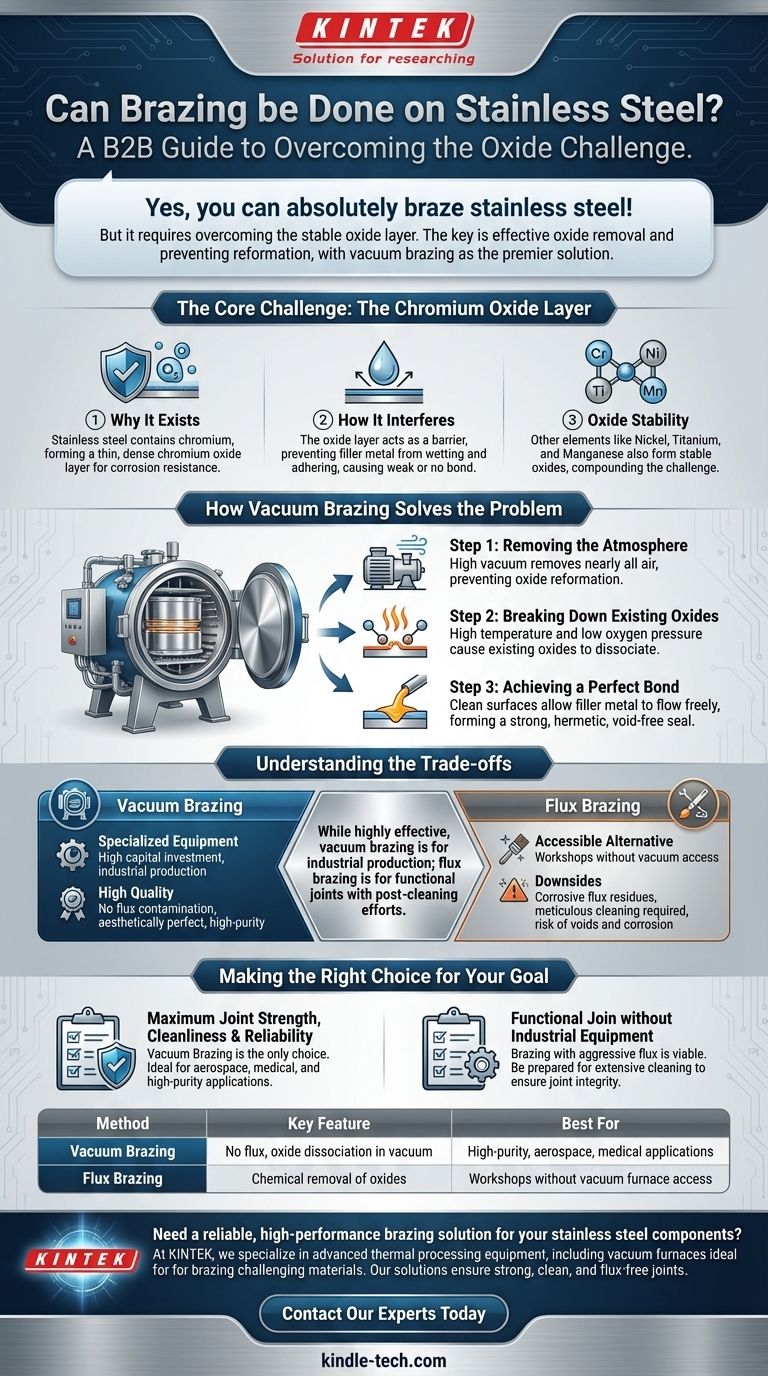
The Core Challenge: The Chromium Oxide Layer
The very property that makes stainless steel "stainless"—its corrosion resistance—is what makes it challenging to braze.
Why This Layer Exists
Stainless steel contains a significant amount of chromium. This element reacts instantly with oxygen in the air to form a thin, dense, and non-reactive layer of chromium oxide. This passive layer protects the underlying steel from further oxidation and corrosion.
How It Interferes with Brazing
Brazing works by creating a metallurgical bond between a filler metal and the base metals. For this to happen, the molten filler metal must "wet" the surface, meaning it must flow and adhere to it.
The oxide layer on stainless steel acts as a physical barrier, preventing the filler metal from making direct contact with the steel. This results in poor wetting, a weak bond, or no bond at all.
The Stability of the Oxides
The challenge is compounded by other elements in stainless steel alloys, such as nickel (Ni), titanium (Ti), and manganese (Mn). These also form very stable oxides that are difficult to remove with conventional methods.
How Vacuum Brazing Solves the Problem
For high-performance applications, vacuum brazing is the definitive method for joining stainless steel. The process systematically neutralizes the oxide problem in a controlled environment.
Step 1: Removing the Atmosphere
The entire brazing process takes place inside a furnace from which nearly all air has been pumped out, creating a high vacuum. Removing oxygen from the environment is critical because it prevents the oxide layer from reforming once it is removed.
Step 2: Breaking Down Existing Oxides
As the stainless steel parts are heated to high temperatures within the vacuum, the existing oxide layer becomes unstable. The combination of high heat and the absence of oxygen pressure causes the oxides to dissociate, or break down, leaving behind a pristine, clean metal surface.
Step 3: Achieving a Perfect Bond
With the oxide barrier gone and no oxygen to reform it, the molten brazing filler metal can flow freely across the clean surfaces. This perfect wetting allows the filler to be drawn into the joint by capillary action, forming a strong, void-free, and hermetic seal upon cooling.
Understanding the Trade-offs
While vacuum brazing is highly effective, it's essential to understand its place among other methods.
The Need for Specialized Equipment
Vacuum brazing is not a manual process. It requires a significant capital investment in a vacuum furnace and sophisticated control systems. This makes it suitable for industrial production and high-value components, not small-scale workshop repairs.
The Alternative: Flux Brazing
The traditional alternative is to use a chemical flux. A highly aggressive flux, often called "black flux" for stainless steel, is applied to the joint. During heating, the flux chemically attacks and dissolves the oxide layer, allowing the brazing filler to wet the surface.
Downsides of Using Flux
While functional, flux presents several disadvantages. The flux residues are corrosive and must be meticulously cleaned after brazing, which can be difficult in complex assemblies. Any trapped flux can lead to voids in the joint or cause corrosion later in the component's service life.
Making the Right Choice for Your Goal
Selecting the correct method depends entirely on your application's requirements for quality, performance, and scale.
- If your primary focus is maximum joint strength, cleanliness, and reliability: Vacuum brazing is the only choice. It produces aesthetically perfect joints with no flux contamination, making it ideal for medical, aerospace, and high-purity applications.
- If your primary focus is a functional join without access to industrial equipment: Brazing with an appropriate aggressive flux is a viable method, but you must be prepared for extensive and thorough post-brazing cleaning to ensure joint integrity.
Ultimately, understanding the properties of stainless steel's oxide layer is the key to successfully joining it.
Summary Table:
| Method | Key Feature | Best For |
|---|---|---|
| Vacuum Brazing | No flux, oxide dissociation in vacuum | High-purity, aerospace, medical applications |
| Flux Brazing | Chemical removal of oxides | Workshops without vacuum furnace access |
Need a reliable, high-performance brazing solution for your stainless steel components?
At KINTEK, we specialize in advanced thermal processing equipment, including vacuum furnaces ideal for brazing challenging materials like stainless steel. Our solutions ensure strong, clean, and flux-free joints for critical applications in aerospace, medical, and other high-tech industries.
Contact our experts today to discuss how we can help you achieve superior brazing results and enhance your manufacturing capabilities.
Visual Guide
Related Products
- Vacuum Heat Treat Sintering Brazing Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Molybdenum Vacuum Heat Treat Furnace
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- Laboratory High Pressure Vacuum Tube Furnace
People Also Ask
- Why KBr is used in FTIR system? The Key to Accurate Mid-IR Spectral Analysis
- What are the 3 examples of heat transfer? Conduction, Convection & Radiation Explained
- Which type of sputtering system will be used to deposit ZnO thin film? Choose Between RF and DC Magnetron Sputtering
- What is the carbon footprint of diamond mining? Uncovering the True Environmental and Ethical Cost
- What are the two types of hot air ovens? Choose the Right Air Circulation for Your Lab
- Is pyrolysis of plastic harmful to the environment? It Depends on How You Manage the Outputs
- Why is it necessary to configure drying equipment before TSA? Boost CO2 Capture Efficiency and Adsorbent Life
- Why plastic pyrolysis is not sustainable? The Hidden Environmental and Economic Costs



















