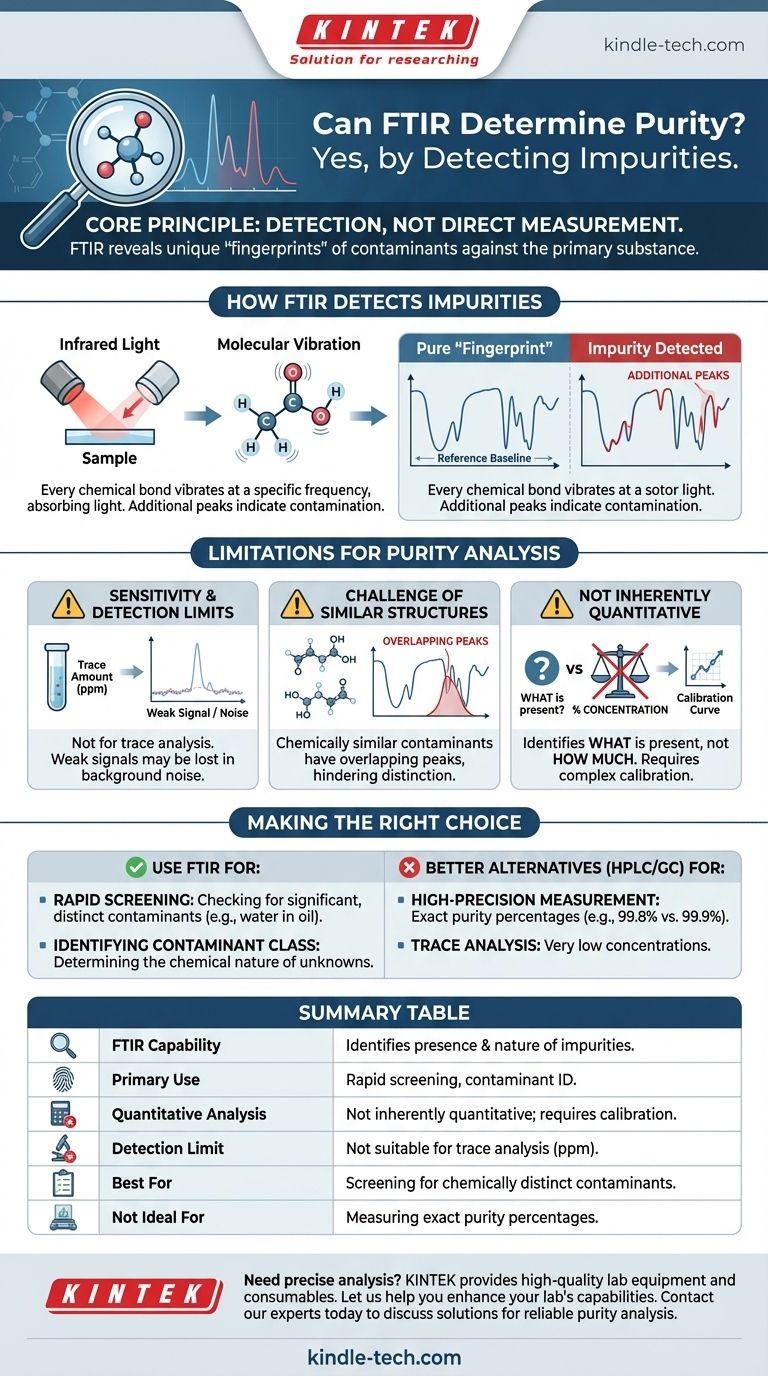
Yes, FTIR can be used to assess purity, but it operates differently than many expect. It excels at identifying the presence and chemical nature of impurities rather than providing a direct percentage of the main component, especially at very high purity levels.
The core principle is one of detection, not direct measurement. FTIR assesses purity by revealing the unique spectral "fingerprints" of contaminants. Its effectiveness depends entirely on whether an impurity's chemical signature is distinct and detectable against the backdrop of the primary substance.
How FTIR Detects Impurities
FTIR, or Fourier Transform Infrared Spectroscopy, works by shining infrared light on a sample and measuring how much light is absorbed at different frequencies. This process provides a spectrum that acts as a unique chemical fingerprint.
The Principle of Molecular Vibrations
Every chemical bond within a molecule (like C-H, O-H, or C=O) vibrates at a specific frequency. When infrared light matching that frequency hits the bond, the energy is absorbed.
FTIR spectroscopy maps these absorptions, creating a plot of peaks that corresponds to the specific chemical bonds present in your sample.
Establishing a Pure "Fingerprint"
For a known, pure substance, the resulting FTIR spectrum is a consistent and recognizable pattern, often called its "fingerprint." This reference spectrum serves as the baseline for purity analysis.
Identifying Contaminant Peaks
An impurity is simply another chemical substance. If it is present in your sample, its own unique chemical bonds will also absorb infrared light.
These absorptions will appear as additional peaks in the spectrum that are not present in the reference fingerprint of the pure material. The presence of these unexpected peaks is a direct indication of contamination.
Understanding the Limitations for Purity Analysis
While powerful, FTIR is not a universal solution for purity measurement. Its limitations are critical to understand before relying on it.
Sensitivity and Detection Limits
FTIR is generally not suited for trace analysis. If an impurity is present at a very low concentration (e.g., parts per million), its absorption signal may be too weak to be distinguished from the background noise of the spectrum.
The Challenge of Similar Structures
If a contaminant is chemically very similar to the main substance (e.g., a similar solvent or a structural isomer), their infrared absorption peaks may overlap significantly. This can make it difficult or impossible to distinguish the impurity's signal from the main component's fingerprint.
It Is Not Inherently Quantitative
Out of the box, FTIR tells you what is present, not necessarily how much. To determine the concentration or percentage of an impurity, you must perform a more complex quantitative analysis. This requires creating a calibration curve using standards with known concentrations of the impurity, a process that can be time-consuming.
Making the Right Choice for Your Goal
Use FTIR for purity analysis when its strengths align with your objective. For high-precision work, other methods are superior.
- If your primary focus is rapid screening for significant contamination: FTIR is an excellent and fast method to verify if a known material is contaminated with a chemically different substance (e.g., checking for water in oil).
- If your primary focus is identifying the nature of an unknown contaminant: FTIR is a powerful tool for determining the class of chemical compound that is acting as the impurity.
- If your primary focus is measuring exact purity percentages (e.g., 99.8% vs. 99.9%): Techniques like High-Performance Liquid Chromatography (HPLC) or Gas Chromatography (GC) are the industry standard and far more appropriate.
Ultimately, FTIR is a valuable tool for purity assessment and contaminant identification, but it is not a direct replacement for true quantitative purity analysis.
Summary Table:
| Aspect | FTIR Capability |
|---|---|
| Primary Use | Identifies the presence and chemical nature of impurities. |
| Quantitative Analysis | Not inherently quantitative; requires calibration for concentration. |
| Detection Limit | Generally not suitable for trace analysis (e.g., parts per million). |
| Best For | Rapid screening for significant, chemically distinct contaminants. |
| Not Ideal For | Measuring exact purity percentages (e.g., 99.8% vs. 99.9%). |
Need precise analysis for your laboratory samples?
FTIR is a powerful tool for identifying contaminants, but achieving accurate, quantitative results often requires the right equipment and expertise. KINTEK specializes in providing high-quality lab equipment and consumables to meet your specific analytical challenges.
Let us help you enhance your lab's capabilities. Contact our experts today to discuss your needs and discover the ideal solutions for reliable purity analysis.
Visual Guide
Related Products
- High Performance Laboratory Stirrers for Diverse Applications
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Laboratory High Pressure Horizontal Autoclave Steam Sterilizer for Lab Use
- Laboratory Multifunctional Small Speed-Adjustable Horizontal Mechanical Shaker for Lab
- Square Lab Press Mold for Laboratory Applications
People Also Ask
- What are the alternatives to rotavap? Find the Right Solvent Removal Method for Your Lab
- What is the sputtering process in metals? A Guide to Precision Thin-Film Deposition
- What role does a magnetic stirrer play in the formulation of Palladium/Graphene slurry for electrode fabrication?
- How does an Ultrafast High-temperature Sintering (UHS) system work? Achieve 3000°C in Seconds
- What is a braze repair process? A Low-Heat Solution for Strong, Seamless Metal Joining
- What temperature should brazing be? Master the Key to Strong, Reliable Joints
- What are the different types of ash test? Choose the Right Method for Your Material
- What is the vacuum sublimation method? A Guide to High-Purity Material Purification



















