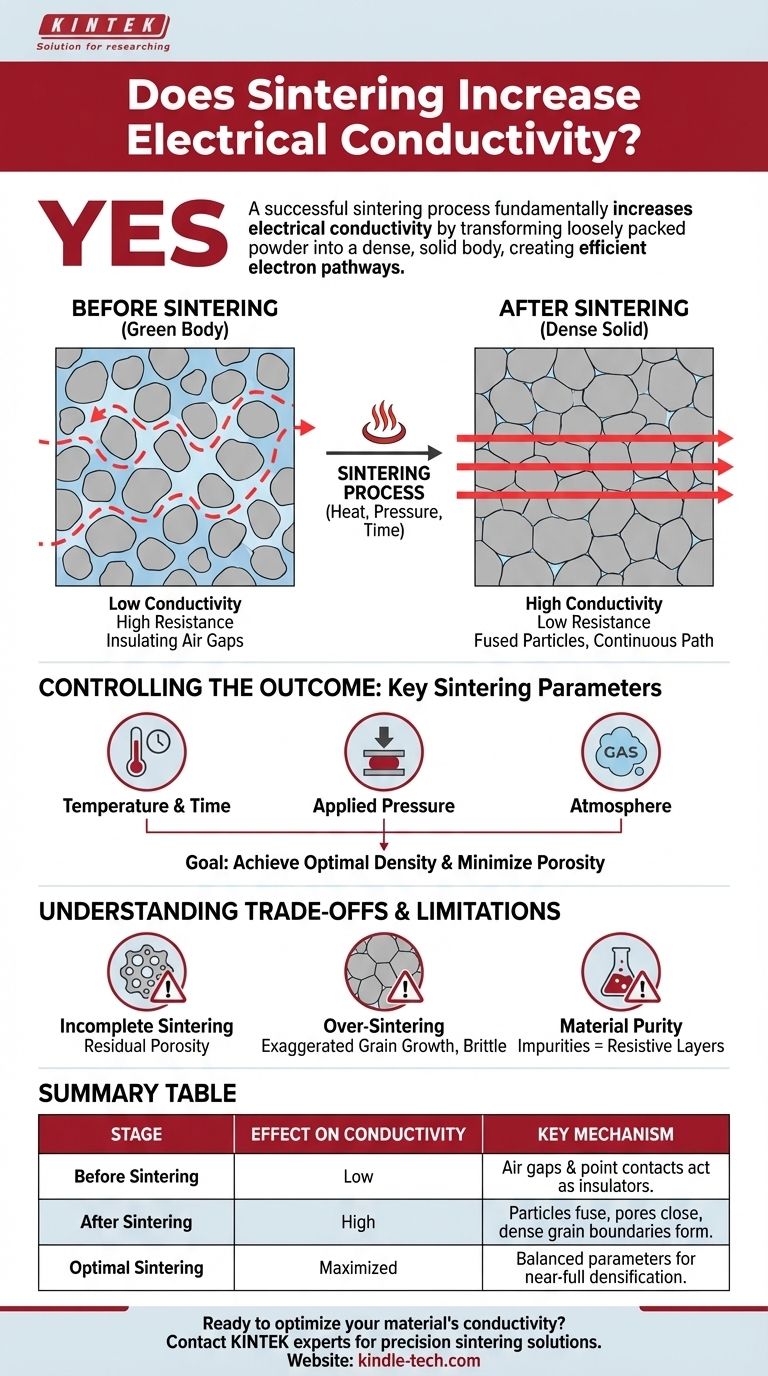
Yes, a successful sintering process fundamentally increases electrical conductivity. This enhancement occurs because sintering transforms a loosely packed, porous powder compact into a dense, solid body with intimate contact between material grains, creating a clear and efficient path for electrons to flow.
The core purpose of sintering is to reduce porosity. By eliminating the insulating air gaps between particles and fusing them together, sintering drastically lowers electrical resistance and creates a continuous, conductive pathway through the material.

The Mechanism: From Powder to Conductive Solid
To understand why conductivity increases, we must first visualize the material before and after sintering. The change at the microscopic level is the entire basis for the improvement in electrical properties.
The Problem with Un-sintered Powder
An un-sintered part, even after being pressed into a shape (a "green body"), is essentially a collection of individual particles with a significant volume of air-filled pores between them.
Electrical current struggles to flow through this structure. The contact points between particles are minuscule, creating high resistance, and the air gaps are effective insulators, forcing electrons to follow a difficult, winding path.
How Sintering Creates a Conductive Pathway
Sintering uses heat (below the material's melting point) to drive material transport, causing the individual particles to bond and fuse together.
This process systematically eliminates the pores. As the particles merge and the gaps between them close, the density of the material increases, and the cross-sectional area available for electron flow grows dramatically.
The Role of Grain Boundaries
The result of sintering is a polycrystalline solid made up of "grains" that are joined at "grain boundaries."
While these boundaries can still present some resistance compared to a perfect single crystal, a well-formed, fused boundary is vastly more conductive than the simple point-to-point contact found in an un-sintered powder.
Controlling the Outcome: Key Sintering Parameters
Achieving higher conductivity isn't automatic; it depends entirely on how the sintering process is controlled. The final properties are a direct result of the parameters you choose.
Critical Process Variables
As outlined in research, several factors are critical. The most fundamental are sintering temperature, holding time, and applied pressure. These variables directly control the rate and extent of densification.
The Goal: Achieving Optimal Density
The primary objective for enhancing conductivity is to achieve the highest possible density, meaning the lowest possible residual porosity.
Careful adjustment of the sintering parameters allows you to control the final microstructure, including the size of the pores and the shape of the grain boundaries, which dictate the material's final performance.
Understanding the Trade-offs and Limitations
Sintering is a powerful process, but improper execution can fail to deliver the desired results or even introduce new problems.
The Risk of Incomplete Sintering
If the temperature is too low or the holding time is too short, the material will not fully densify. This leaves behind residual porosity, which will significantly limit the final electrical conductivity.
The Danger of Over-sintering
Conversely, using excessive temperatures or times can lead to exaggerated grain growth. While this might reduce pore volume, it can severely degrade other critical properties, such as mechanical strength and durability.
The Influence of Material Purity
The conductivity of the final part is also highly dependent on the purity of the initial powder. During heating, impurities can migrate to the newly forming grain boundaries, creating resistive layers that impede electron flow even in a fully dense material.
Making the Right Choice for Your Goal
To effectively use sintering, you must align your process parameters with your primary objective for the final component.
- If your primary focus is maximizing conductivity: Your goal is to achieve near-full densification by carefully optimizing temperature, pressure, and time to eliminate porosity.
- If your primary focus is balancing conductivity with mechanical strength: You must prevent excessive grain growth by avoiding overly high temperatures or extended holding times, which can cause brittleness.
- If you are experiencing inconsistent results: Methodically analyze your process, paying close attention to temperature uniformity, heating rates, and atmospheric conditions, as these directly control the final microstructure.
Ultimately, viewing sintering as a precise tool for microstructural engineering is the key to reliably controlling your material's final conductivity.
Summary Table:
| Sintering Stage | Effect on Conductivity | Key Mechanism |
|---|---|---|
| Before Sintering | Low | Air gaps and point contacts between particles act as insulators. |
| After Sintering | High | Particles fuse, pores close, and dense grain boundaries form conductive pathways. |
| Optimal Sintering | Maximized | Achieved by balancing temperature, time, and pressure for near-full densification. |
Ready to optimize your material's conductivity? At KINTEK, we specialize in precision lab equipment and consumables for sintering processes. Whether you're working with metals, ceramics, or advanced composites, our solutions help you achieve the perfect balance of density, conductivity, and mechanical strength. Contact our experts today to discuss how we can support your laboratory's sintering needs and enhance your material performance.
Visual Guide
Related Products
- Vacuum Heat Treat and Sintering Furnace with 9MPa Air Pressure
- Dental Porcelain Zirconia Sintering Ceramic Vacuum Press Furnace
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
- Spark Plasma Sintering Furnace SPS Furnace
- 600T Vacuum Induction Hot Press Furnace for Heat Treat and Sintering
People Also Ask
- What is the difference between electric arc furnace and plasma arc furnace? Choose the Right Tool for Your Heat Processing Needs
- What is the difference between smelting and sintering? A Guide to Metal Extraction vs. Powder Consolidation
- What is pull down time in ULT freezers and what factors affect it? Ensure Your Sample Security with Efficient Cooling
- What types of furnaces are used for sintering ceramics? Choose the Right Kiln for Your Production
- What is the primary role of an electric heating constant temperature blast drying oven in transparent wood prep?
- Is pyrolysis of plastic environmentally friendly? A Deep Dive into the Green Potential and Risks
- Why is it necessary to use a vacuum drying oven for COF powders? Maximize Porosity & Material Stability
- What are the pros and cons of hot forging? Unlock Superior Strength for Critical Components



















