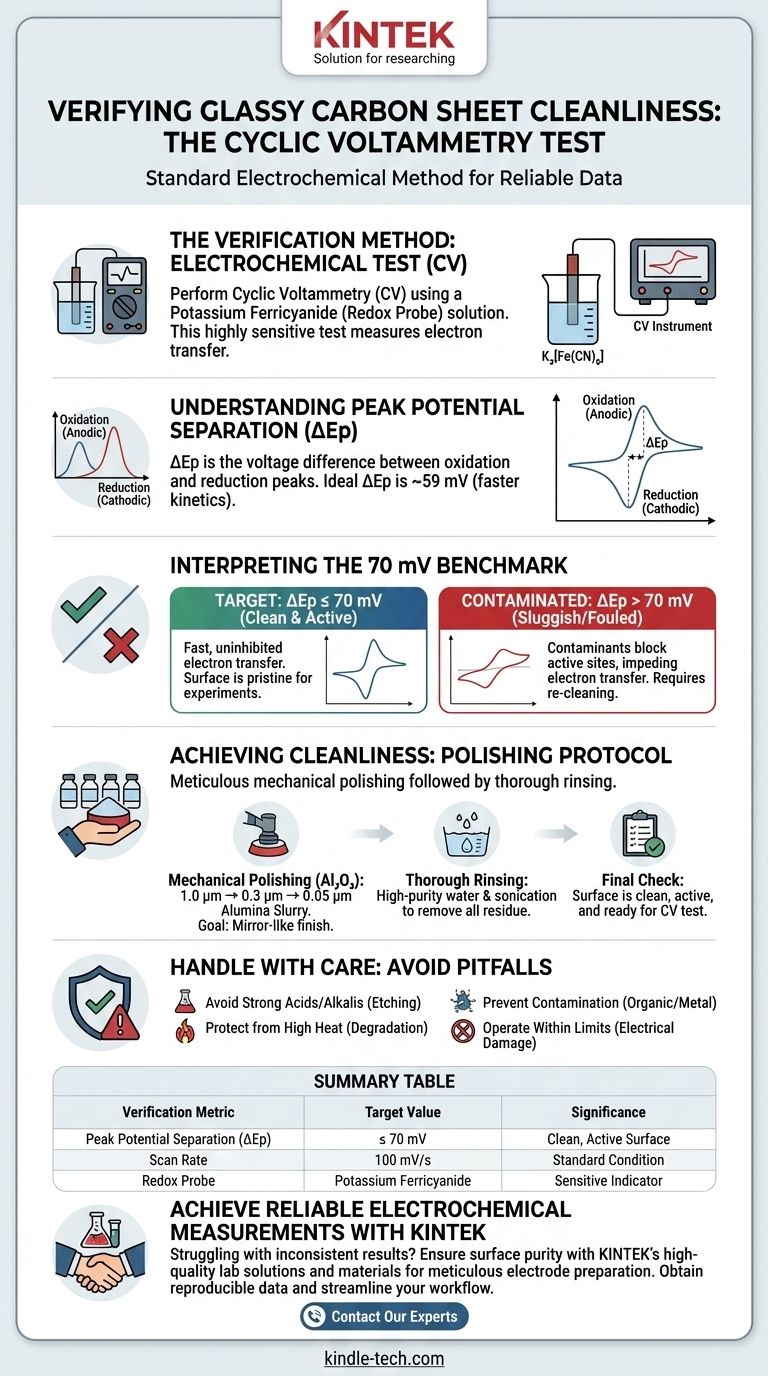
To verify the cleanliness of a pre-treated glassy carbon sheet, you must perform an electrochemical test using cyclic voltammetry (CV). The standard method involves using a potassium ferricyanide solution and measuring the separation between the oxidation and reduction peaks. A clean surface is indicated by a peak potential separation (ΔEp) of less than or equal to 70 mV at a scan rate of 100 mV/s.
A low peak potential separation in a ferricyanide redox probe signifies fast, uninhibited electron transfer, which is the electrochemical signature of a truly clean and active glassy carbon surface. This quantitative test is the industry standard for confirming that your pre-treatment protocol was successful.
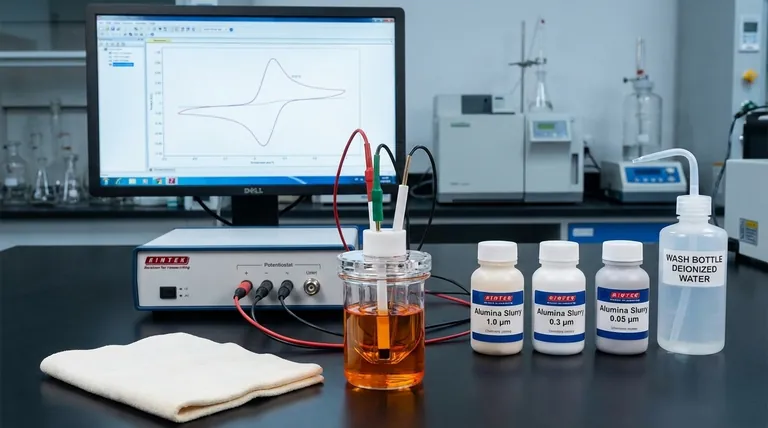
Understanding the Verification Method
The goal of pre-treatment is to create a pristine surface for your experiment. The CV test in potassium ferricyanide provides a clear, quantitative measure of how well you have achieved this.
Why Potassium Ferricyanide?
Potassium ferricyanide, K₃[Fe(CN)₆], is an ideal redox probe. Its reaction mechanism is a simple, single-electron transfer process that is highly sensitive to the state of the electrode surface.
A clean surface allows for rapid electron transfer, while any contamination or fouling will slow it down, which is directly observable in the CV results.
What is Peak Potential Separation (ΔEp)?
Peak potential separation, or ΔEp, is the difference in voltage between the anodic (oxidation) peak and the cathodic (reduction) peak on your cyclic voltammogram.
For a perfectly reversible, one-electron process, the theoretical ΔEp is approximately 59 mV. The closer your measurement is to this ideal value, the faster the electron transfer kinetics.
Interpreting the 70 mV Benchmark
A measured ΔEp of 70 mV or less indicates that the electron transfer is fast and nearly reversible. This is strong evidence that the glassy carbon surface is clean and electrochemically active.
If your ΔEp is significantly higher than 70 mV, it suggests a "sluggish" or contaminated surface. This means contaminants are blocking active sites and impeding electron transfer, rendering the electrode unsuitable for sensitive measurements.
Achieving a Clean Surface: The Polishing Protocol
Verification is the final step; proper preparation is what ensures a good result. Achieving a clean surface requires a meticulous mechanical polishing process.
Mechanical Polishing
The standard procedure involves polishing the glassy carbon sheet on a soft pad, like chamois cloth, with a sequence of alumina (Al₂O₃) suspensions.
You must polish with progressively finer grits. A typical sequence is 1.0 µm, followed by 0.3 µm, and finally 0.05 µm alumina slurry. This process removes microscopic imperfections and contaminants.
The goal is to produce a mirror-like finish with no scratches visible to the naked eye.
Thorough Rinsing
After the final polishing step, the surface will be covered in alumina residue. It is critical to rinse the sheet thoroughly with high-purity water (e.g., deionized water) to remove all particles.
For best results, sonication in a beaker of pure water for a few minutes is highly effective at dislodging any remaining polishing debris from microscopic crevices.
Understanding the Trade-offs and Pitfalls
A clean glassy carbon surface is highly active and prone to re-contamination or damage if not handled correctly.
Avoid Chemical and Physical Damage
Do not immerse the sheet in strong acid or strong alkali solutions for extended periods. This can etch and permanently damage the carbon structure.
Likewise, protect the sheet from high-temperature sources. Overheating can alter the surface and degrade its performance.
Prevent Contamination
Once cleaned, the surface is highly susceptible to contamination from organic substances and metal compounds. Always work in a clean environment and use high-purity solvents and reagents.
Even brief exposure to a contaminated environment can be enough to foul the electrode and increase your ΔEp.
Operate Within Electrical Limits
When performing electrochemical experiments, always operate within the specified current and voltage limits for your electrode. Exceeding these limits, particularly by applying extreme potentials, can cause irreversible damage to the surface.
How to Apply This to Your Project
Use these guidelines to ensure your glassy carbon electrodes are prepared correctly for reliable and reproducible measurements.
- If your primary focus is obtaining reproducible data: Always perform the ferricyanide CV test before a critical experiment to confirm your electrode's activity is within the acceptable range (ΔEp ≤ 70 mV).
- If you observe a high peak separation (> 70 mV): Your surface is not sufficiently clean. You must repeat the entire polishing and rinsing protocol before re-testing.
- If you consistently struggle with high ΔEp values: Systematically evaluate your entire experimental setup for sources of contamination, including impure solvents, unclean glassware, or even airborne vapors in the lab.
A clean, well-characterized electrode is the foundation of trustworthy electrochemical results.
Summary Table:
| Verification Metric | Target Value | Significance |
|---|---|---|
| Peak Potential Separation (ΔEp) | ≤ 70 mV | Indicates a clean, electrochemically active surface |
| Scan Rate | 100 mV/s | Standard condition for the test |
| Redox Probe | Potassium Ferricyanide | Sensitive indicator of surface contamination |
Achieve Reliable Electrochemical Measurements with KINTEK
Struggling with inconsistent results or contaminated electrodes? Proper surface preparation is the foundation of accurate electrochemical data. KINTEK specializes in high-quality lab equipment and consumables, including materials essential for meticulous electrode preparation.
Our products help you:
- Ensure Surface Purity: Access high-purity alumina polishing powders and clean lab supplies to minimize contamination risks.
- Obtain Reproducible Data: Achieve the mirror-like finish and low ΔEp values necessary for trustworthy experiments.
- Streamline Your Workflow: From polishing to verification, trust equipment designed for precision and repeatability.
Let's optimize your electrode preparation process. Contact our experts today to discuss how KINTEK's solutions can enhance your laboratory's electrochemical capabilities and data quality.
Visual Guide
Related Products
- Glassy Carbon Electrochemical Electrode
- Glassy Carbon Sheet RVC for Electrochemical Experiments
- Electrolytic Electrochemical Cell for Coating Evaluation
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Gold Disc Electrode
People Also Ask
- What are the functions of a glassy carbon electrode in CV testing of antioxidants? Enhance Your Redox Analysis Accuracy
- Why is glassy carbon selected for mediator-assisted indirect oxidation of glycerol? The Key to Unbiased Research
- What is a glassy carbon electrode made of? The Engineered Material Powering Electrochemical Analysis
- How is a glassy carbon electrode activated before an experiment? Achieve Clean, Reproducible Electrochemical Data
- What are the pre-treatment steps for a glassy carbon electrode before use? Ensure Reliable Electrochemical Data



















