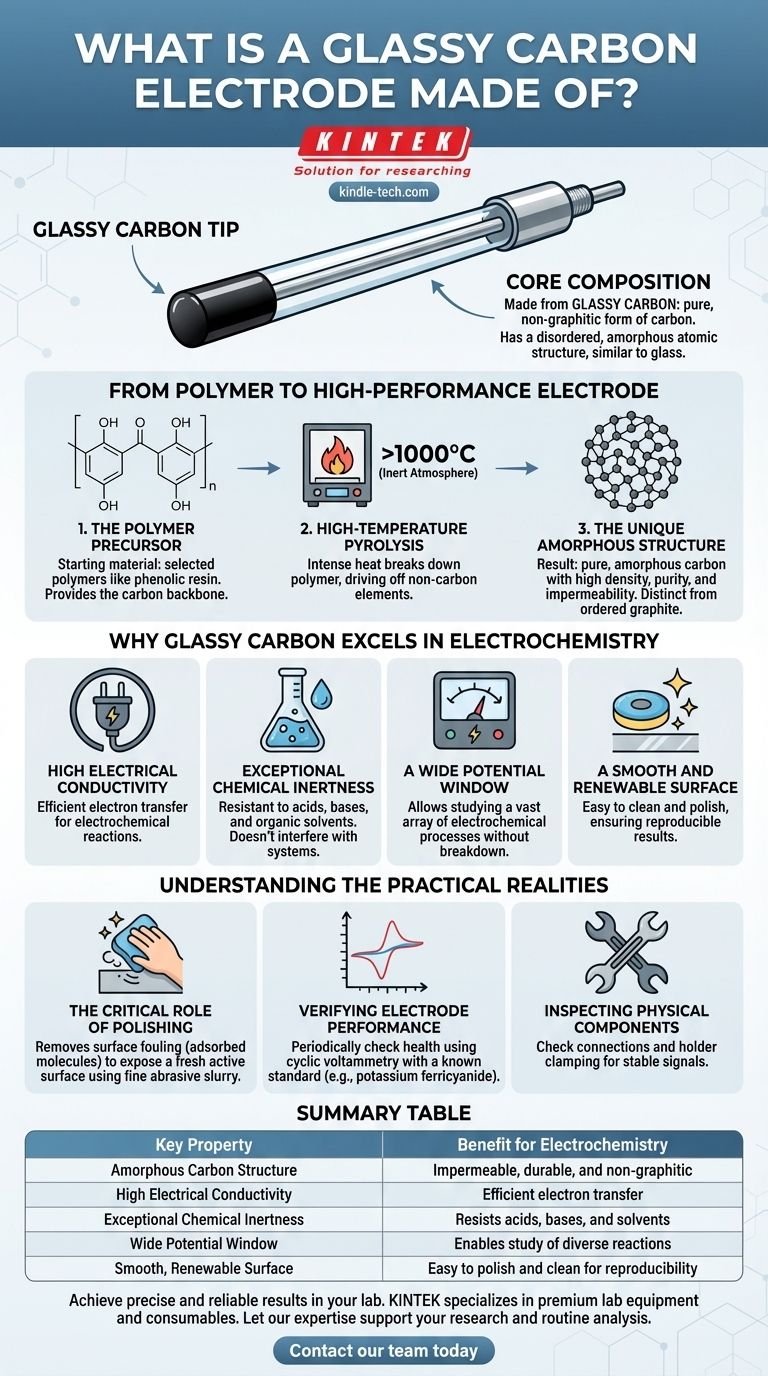
At its core, a glassy carbon electrode is made from glassy carbon, a unique, non-graphitic form of pure carbon. Unlike the ordered crystalline structure of graphite, glassy carbon has a disordered, amorphous atomic structure, similar to that of glass. This material is synthesized by subjecting specific polymers, such as phenolic resin, to extremely high temperatures in a controlled, inert environment.
The choice of glassy carbon is deliberate; its unique, glass-like atomic structure provides a rare combination of electrical conductivity, extreme chemical resistance, and a smooth, renewable surface, making it a benchmark standard for a wide range of electrochemical applications.

From Polymer to High-Performance Electrode
The properties of a glassy carbon electrode are a direct result of its manufacturing process. This isn't a naturally occurring material but a highly engineered one designed for specific performance characteristics.
The Polymer Precursor
The process begins with a carefully selected polymer precursor, most commonly a phenolic resin. This starting material provides the carbon backbone that will eventually form the final product.
High-Temperature Pyrolysis
This polymer is then subjected to a process called pyrolysis, where it is heated to very high temperatures (often exceeding 1000°C) in an inert atmosphere. This intense heat breaks down the polymer, driving off non-carbon elements and leaving behind a structure of pure, amorphous carbon.
The Unique Amorphous Structure
The result is a material with a disordered, glass-like structure. It has high density, high purity, and is impermeable to gases. This structure is fundamentally different from graphite, which consists of ordered layers that can flake apart.
Why Glassy Carbon Excels in Electrochemistry
The physical structure of glassy carbon gives it a set of properties that make it an almost ideal material for working electrodes in research and analysis.
High Electrical Conductivity
Like other forms of carbon, glassy carbon conducts electricity well. This is a fundamental requirement for any electrode material, allowing for efficient electron transfer during electrochemical reactions.
Exceptional Chemical Inertness
Glassy carbon is extremely resistant to chemical attack. It does not readily react with acids, bases, or organic solvents, which ensures that the electrode itself does not interfere with the chemical system being studied.
A Wide Potential Window
This inertness leads to a wide potential window. The electrode can be subjected to a broad range of positive and negative voltages without breaking down or reacting, allowing researchers to study a vast array of electrochemical processes.
A Smooth and Renewable Surface
The surface of a glassy carbon electrode is smooth and easily cleaned. This is a crucial practical advantage, as the surface can be polished to remove contaminants and restore a pristine, highly reproducible state for new experiments.
Understanding the Practical Realities
While robust, a glassy carbon electrode is a precision instrument that requires proper maintenance to deliver accurate and repeatable results. Its performance is entirely dependent on the condition of its surface.
The Critical Role of Polishing
Over time, molecules from the solution can adsorb onto the electrode's surface, a process known as fouling. This deactivates the electrode and degrades its performance. Regular polishing with a fine abrasive slurry (like alumina) is essential to remove these layers and expose a fresh, active surface.
Verifying Electrode Performance
It is standard practice to periodically verify the electrode's health. This is typically done using cyclic voltammetry with a well-understood chemical system, such as potassium ferricyanide, to ensure the electrode is behaving as expected.
Inspecting Physical Components
Beyond the carbon surface, the electrode's physical housing is also important. Periodically checking wire connections and the clamping force of the holder ensures a stable electrical connection, preventing noise and errors in measurement.
Making the Right Choice for Your Goal
Properly using a glassy carbon electrode means aligning its maintenance with your experimental needs.
- If your primary focus is routine analysis: Regular polishing before each set of experiments is the key to achieving consistent and reliable data.
- If your primary focus is sensor development: The smooth, easily cleaned surface provides a perfect substrate for modification with catalysts, enzymes, or other materials.
- If your primary focus is fundamental research: The wide potential window and chemical inertness offer a reliable and non-interfering baseline for studying novel electrochemical systems.
Understanding that this material's performance is tied directly to its pristine surface is the first step toward mastering your electrochemical measurements.
Summary Table:
| Key Property | Benefit for Electrochemistry |
|---|---|
| Amorphous Carbon Structure | Impermeable, durable, and non-graphitic |
| High Electrical Conductivity | Efficient electron transfer |
| Exceptional Chemical Inertness | Resists acids, bases, and solvents |
| Wide Potential Window | Enables study of diverse reactions |
| Smooth, Renewable Surface | Easy to polish and clean for reproducibility |
Achieve precise and reliable results in your lab. The performance of your electrochemical analysis depends on high-quality electrodes and equipment. KINTEK specializes in premium lab equipment and consumables, including materials essential for electrode fabrication and maintenance. Let our expertise support your research and routine analysis. Contact our team today to discuss your specific laboratory needs.
Visual Guide
Related Products
- Glassy Carbon Electrochemical Electrode
- Glassy Carbon Sheet RVC for Electrochemical Experiments
- Reference Electrode Calomel Silver Chloride Mercury Sulfate for Laboratory Use
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Graphite Disc Rod and Sheet Electrode Electrochemical Graphite Electrode
People Also Ask
- What is the typical working electrode potential range for a glassy carbon electrode in aqueous electrolytes? A Guide to Accurate Electrochemical Measurements
- How is a glassy carbon electrode activated before an experiment? Achieve Clean, Reproducible Electrochemical Data
- How should a glassy carbon electrode be stored during long periods of non-use? Ensure Peak Performance & Longevity
- What is the proper post-treatment and storage procedure for a glassy carbon electrode? Ensure Reliable, Reproducible Results
- How to make a glassy carbon electrode? A Guide to the Industrial Pyrolysis Process



















