The most common shape for a glassy carbon electrode (GCE) is a flat, circular disc. These discs are typically available in standard diameters of 2mm, 3mm, 4mm, and 5mm, embedded in an insulating sheath. The entire assembly is usually manufactured in either a straight "rod" configuration or an L-shaped configuration for specific electrochemical cell geometries.
While the standard disc shape provides a foundation for reproducible experiments, the true power of the glassy carbon electrode stems from its material properties—combining chemical inertness and high conductivity to make it a reliable workhorse for modern electrochemistry.
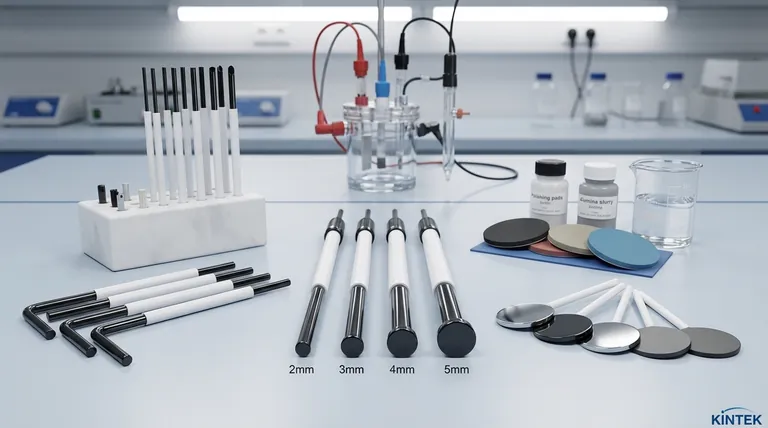
The Anatomy of a Glassy Carbon Electrode
To understand how to use a GCE effectively, it's important to recognize why it's designed the way it is. The shape, size, and configuration all serve specific purposes in an electrochemical setup.
The Standard Disc Shape
The disc shape is the default for a crucial reason: it provides a well-defined, planar surface area. This is essential for quantitative analysis, as many fundamental electrochemical equations rely on a known, consistent electrode area to calculate results accurately.
Common Diameter Sizes
The diameter of the disc—most often 2mm, 3mm, 4mm, or 5mm—is a key variable. A smaller diameter can be beneficial for certain applications like microanalysis, while a larger diameter provides a stronger overall signal, which can be useful for bulk analysis.
Straight vs. L-Shaped Configurations
The physical form of the electrode body is primarily a matter of practicality. A straight configuration is a simple rod, suitable for most standard electrochemical cells. An L-shaped configuration is designed for cells where vertical space is limited or to avoid interference with other components like reference electrodes or gas bubblers.
Why Glassy Carbon is the Material of Choice
Glassy carbon, sometimes called vitreous carbon, is not just a random choice. It is an advanced, amorphous form of carbon produced by the high-temperature pyrolysis of polymers, giving it a unique combination of properties ideal for an electrode.
Exceptional Chemical Inertness
A GCE is highly resistant to chemical attack and exhibits a wide potential window. This means it remains stable and does not react with the solvent or analyte across a broad range of applied voltages, ensuring the measurements reflect the chemistry you are studying, not the electrode itself.
High Electrical Conductivity
Despite being a form of carbon, its electrical conductivity is excellent, often compared to that of metals. This property ensures efficient electron transfer, which is the fundamental basis of all electrochemical measurements.
A Renewable, Polishable Surface
One of the most practical advantages is its renewable surface. The material is hard and non-porous, allowing it to be polished with an alumina slurry. This process removes surface contaminants and passivated layers, restoring a clean, active surface for highly reproducible results experiment after experiment.
Hardness and Durability
Glassy carbon is extremely hard, approaching the hardness of a diamond, and can withstand very high temperatures (up to 3400°C in a vacuum). This makes it a physically robust and durable tool for long-term lab use.
Understanding the Trade-offs and Best Practices
While highly effective, a glassy carbon electrode is not without its operational requirements. Understanding these nuances is key to achieving reliable data.
Surface Preparation is Critical
The single most important factor for success with a GCE is proper polishing. An improperly cleaned or polished electrode surface is the most common source of poor or non-reproducible results. The goal is to achieve a mirror-like finish free of scratches and residue.
Sensitivity to Fouling
The surface of the electrode can become "fouled" or "passivated" if reaction products or other substances from the solution adsorb onto it. This blocks active sites and inhibits electron transfer. Regular polishing between experiments is the primary way to mitigate this.
Brittleness of the Material
Although very hard, glassy carbon is also brittle. Dropping the electrode or striking the tip against a hard surface can cause it to chip or crack, rendering it unusable. Careful handling is essential.
Selecting the Right Electrode for Your Experiment
Your choice of electrode size and shape should be directly informed by your experimental goals.
- If your primary focus is routine analysis or teaching: A standard 3mm or 5mm straight disc electrode is a versatile and reliable choice for a wide range of applications.
- If your primary focus is detecting low-concentration analytes: A smaller diameter electrode (e.g., 2mm) can offer a better signal-to-noise ratio in certain voltammetric techniques.
- If your primary focus is working with custom or space-constrained cells: An L-shaped configuration or even a custom-designed electrode may be necessary to fit your specific apparatus.
Choosing the right electrode is the first step toward generating clean, reliable, and reproducible electrochemical data.
Summary Table:
| Feature | Common Options | Purpose |
|---|---|---|
| Shape | Flat Disc | Provides a well-defined, planar surface area for quantitative analysis. |
| Diameter | 2mm, 3mm, 4mm, 5mm | Smaller for microanalysis; larger for stronger signal in bulk analysis. |
| Configuration | Straight Rod, L-Shaped | Rod for standard cells; L-shaped for space-constrained geometries. |
Ready to achieve superior electrochemical results?
Choosing the right glassy carbon electrode is critical for the accuracy and reproducibility of your experiments. KINTEK specializes in high-quality lab equipment and consumables, providing the durable, chemically inert electrodes your laboratory needs.
Our range of glassy carbon electrodes, available in standard and custom configurations, ensures you have the perfect tool for applications from routine analysis to sensitive micro-detection.
Let our experts help you select the ideal electrode for your specific setup. Contact KINTEK today to discuss your requirements and enhance your lab's capabilities!
Visual Guide

Related Products
- Glassy Carbon Electrochemical Electrode
- Glassy Carbon Sheet RVC for Electrochemical Experiments
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Gold Disc Electrode
- Reference Electrode Calomel Silver Chloride Mercury Sulfate for Laboratory Use
People Also Ask
- What is the proper post-treatment and storage procedure for a glassy carbon electrode? Ensure Reliable, Reproducible Results
- What are the functions of a glassy carbon electrode in CV testing of antioxidants? Enhance Your Redox Analysis Accuracy
- What is the typical working electrode potential range for a glassy carbon electrode in aqueous electrolytes? A Guide to Accurate Electrochemical Measurements
- How is a glassy carbon electrode activated before an experiment? Achieve Clean, Reproducible Electrochemical Data
- How should a glassy carbon electrode be stored during long periods of non-use? Ensure Peak Performance & Longevity



















