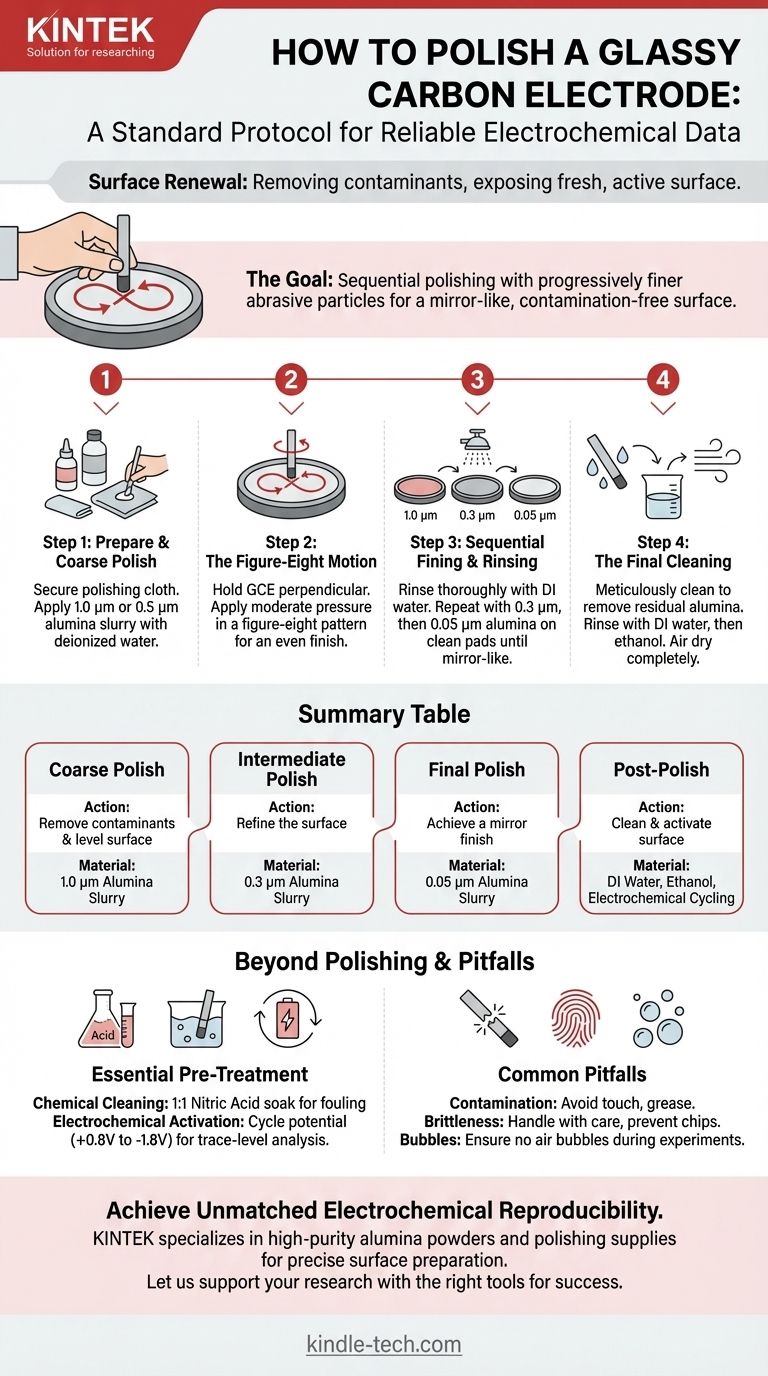
To properly polish a glassy carbon electrode, you must use a sequential process with progressively finer abrasive particles. Start by applying a slurry of alumina powder (e.g., 1.0 µm) and deionized water to a polishing cloth. Holding the electrode perpendicular to the cloth, polish the surface using a figure-eight motion, then thoroughly rinse. Repeat this process with finer grits (e.g., 0.3 µm, then 0.05 µm) until a contamination-free, mirror-like surface is achieved.
The goal of polishing is not simply to make the electrode shiny. It is a critical surface renewal process designed to remove adsorbed contaminants and expose a fresh, reproducible, and electrochemically active surface, which is the foundation of all reliable voltammetric measurements.

The Standard Polishing Protocol: A Step-by-Step Guide
Proper mechanical polishing is a systematic procedure. Rushing this process or skipping steps is the most common source of inconsistent experimental results.
Step 1: Prepare Your Polishing Station
Secure a polishing cloth (e.g., chamois or a designated nylon/silk pad) to a flat, stable surface like a glass plate.
Place a small amount of your coarsest alumina powder (typically 1.0 µm or 0.5 µm) on the cloth. Add a few drops of high-purity or deionized water to create a thin, consistent paste or slurry.
Step 2: The Polishing Motion
Hold the glassy carbon electrode (GCE) so that its polished surface is perfectly perpendicular to the polishing pad. This ensures an even, flat finish.
Apply moderate pressure and move the electrode in a figure-eight pattern. This motion prevents the formation of deep, unidirectional grooves and promotes a more uniform surface.
Step 3: Sequential Fining and Rinsing
After polishing for a minute or two with the first grit, thoroughly rinse the electrode surface with deionized water to remove all abrasive particles. A brief sonication in deionized water is highly effective here.
Move to a clean section of the pad or a new pad. Apply the next-finest grit of alumina (e.g., 0.3 µm) and repeat the polishing and rinsing process.
Finally, repeat the process one last time with the finest powder, typically 0.05 µm alumina, to achieve the final mirror-like finish. The surface should be perfectly smooth with no discernible scratches under good lighting.
Step 4: The Final Cleaning
After the final polish, the electrode must be meticulously cleaned to remove any residual alumina particles, which are electrochemically insulating.
Thoroughly rinse with deionized water, followed by a rinse with ethanol. Allow the electrode to air dry completely before use.
Beyond Polishing: Essential Pre-Treatment
For many sensitive applications, mechanical polishing alone is insufficient. The surface must also be chemically or electrochemically activated to ensure optimal performance.
Why Polishing Is Not Enough
Mechanical polishing creates a clean slate but does not guarantee the surface is in its most electrochemically active state. Contaminants can be introduced from the air, handling, or even the polishing slurry itself.
Chemical Cleaning Methods
Before polishing a heavily fouled electrode, it can be beneficial to soak it. Common methods include immersion in 1:1 nitric acid or a mixture of ammonia water and ethanol. Always rinse the electrode thoroughly with deionized water after any chemical treatment.
Electrochemical Activation
For the highest level of performance, an electrochemical cleaning step is often required after polishing. This typically involves repeatedly cycling the electrode's potential in a supporting electrolyte (e.g., between +0.8V and -1.8V). This process helps to remove any final, trace-level organic contaminants and fully activate the carbon surface.
Common Pitfalls to Avoid
The glassy carbon material is robust but not indestructible. Mishandling it can permanently damage the electrode and compromise your data.
The Dangers of Surface Contamination
The GCE surface is easily contaminated by organic molecules, grease from fingers, or metallic compounds. This contamination can block active sites and severely affect measurements, leading to poor peak shape and reduced currents.
The Brittleness of Glassy Carbon
Glassy carbon is a hard but brittle material. Handle it with care to avoid dropping it or colliding it with hard surfaces. Scratches and chips create surface defects that are difficult to polish away and can lead to erratic electrochemical behavior.
Avoiding Overheating and Bubbles
Never use the electrode at high temperatures, as this can alter the carbon structure. During experiments, ensure no air bubbles adhere to the electrode surface, as this effectively reduces the active electrode area and leads to inaccurate results.
Making the Right Choice for Your Goal
The required level of preparation depends entirely on the demands of your experiment.
- If your primary focus is routine analysis or student labs: A standard mechanical polish from 1.0 µm down to 0.05 µm alumina, followed by a thorough rinse, is typically sufficient.
- If you are working with sensitive, trace-level analytes: Always follow mechanical polishing with an electrochemical activation step to ensure maximum sensitivity and reproducibility.
- If your electrode is heavily fouled or has been stored improperly: Begin with a chemical cleaning step (e.g., nitric acid soak) before proceeding with the full mechanical polishing protocol.
Consistent and meticulous electrode preparation is the foundation of reliable electrochemical data.
Summary Table:
| Polishing Step | Key Action | Recommended Material |
|---|---|---|
| Step 1: Coarse Polish | Remove contaminants and level surface | 1.0 µm Alumina Slurry |
| Step 2: Intermediate Polish | Refine the surface | 0.3 µm Alumina Slurry |
| Step 3: Final Polish | Achieve a mirror finish | 0.05 µm Alumina Slurry |
| Post-Polish | Clean and activate surface | Deionized Water, Ethanol, Electrochemical Cycling |
Achieve Unmatched Electrochemical Reproducibility
Proper electrode preparation is the foundation of reliable data. KINTEK specializes in high-purity laboratory supplies, including the precise alumina powders and polishing materials essential for perfecting your glassy carbon electrode surfaces. Our products are designed to meet the rigorous demands of electrochemical research, ensuring your experiments start with a clean slate.
Let us support your research with the right tools for success.
Contact our lab experts today to find the perfect consumables for your electrode polishing protocol and enhance your lab's performance.
Visual Guide
Related Products
- Glassy Carbon Electrochemical Electrode
- Glassy Carbon Sheet RVC for Electrochemical Experiments
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Reference Electrode Calomel Silver Chloride Mercury Sulfate for Laboratory Use
- Gold Disc Electrode
People Also Ask
- Why is a glassy carbon disc electrode an indispensable consumable? Ensure Reliable Catalyst Evaluation Today
- What is the typical working electrode potential range for a glassy carbon electrode in aqueous electrolytes? A Guide to Accurate Electrochemical Measurements
- What is a glassy carbon electrode made of? The Engineered Material Powering Electrochemical Analysis
- What are the functions of a glassy carbon electrode in CV testing of antioxidants? Enhance Your Redox Analysis Accuracy
- How to make a glassy carbon electrode? A Guide to the Industrial Pyrolysis Process



















