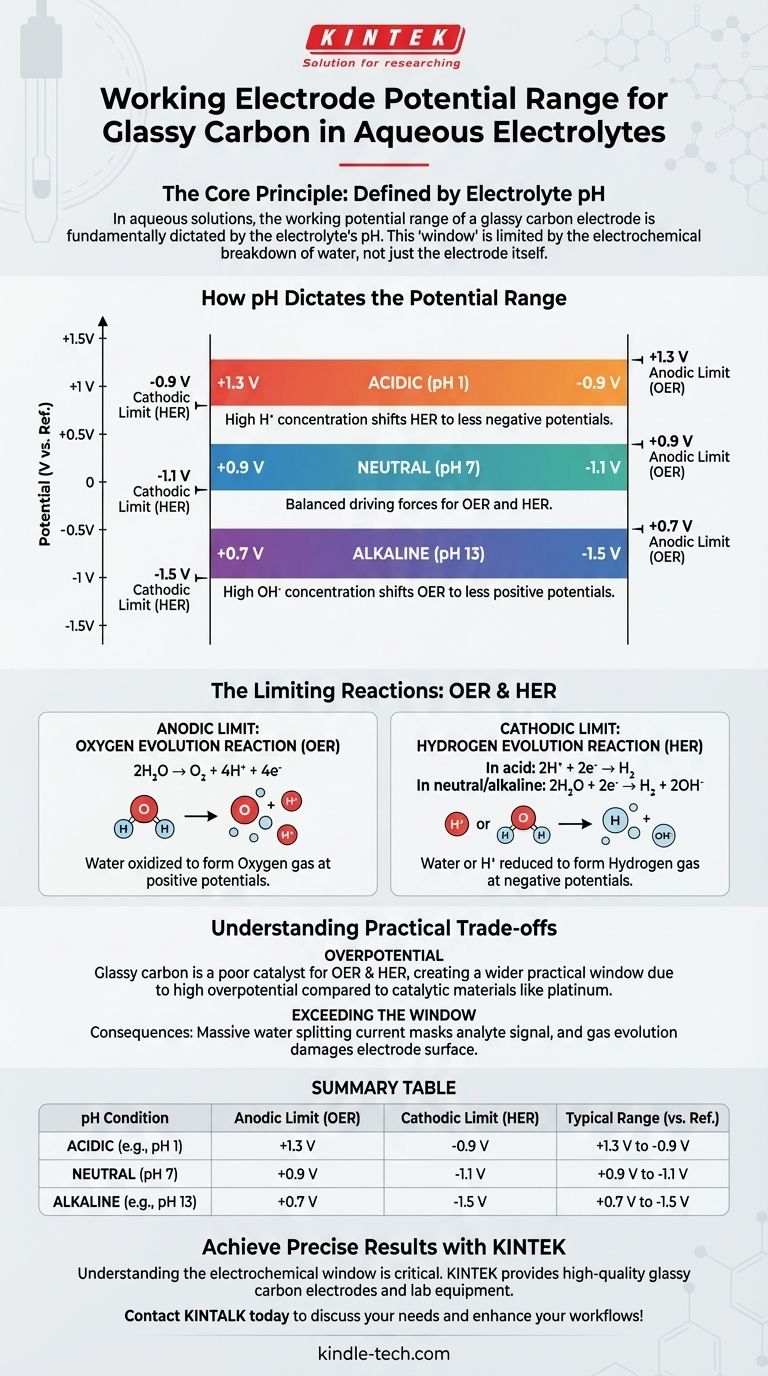
In aqueous solutions, the working potential range for a glassy carbon electrode is fundamentally dictated by the pH of the electrolyte. For acidic solutions, the typical range is +1.3V to -0.9V vs. a standard reference electrode. This shifts to approximately +0.9V to -1.1V in neutral media and +0.7V to -1.5V in alkaline conditions.
The core principle to understand is that the usable potential window is not a property of the electrode alone. It is defined by the electrochemical stability of the solvent—in this case, water—whose breakdown potentials for hydrogen and oxygen evolution are directly dependent on pH.

The "Working Window": Your Zone of Measurement
In electrochemistry, the working potential window (or solvent window) is the range of potentials where the electrolyte and the electrode are themselves inert.
Why This Window is Critical
Within this window, any measured current can be attributed to your analyte of interest. Outside of it, the overwhelming current comes from the decomposition of water, masking your signal and potentially damaging the electrode.
The Boundaries are Set by Water
The limits of this window are defined by two key electrochemical reactions involving water: the oxygen evolution reaction (OER) at the positive end and the hydrogen evolution reaction (HER) at the negative end.
The Limiting Reactions: OER and HER
The potential at which water breaks down is not fixed. It is governed by thermodynamics and is highly sensitive to the concentration of protons (H⁺), which is what we measure as pH.
Anodic Limit: The Oxygen Evolution Reaction (OER)
At sufficiently positive potentials, water is oxidized to form oxygen gas. This reaction marks the positive, or anodic, edge of your working window. The reaction is:
2H₂O → O₂ + 4H⁺ + 4e⁻
Cathodic Limit: The Hydrogen Evolution Reaction (HER)
At sufficiently negative potentials, water (or H⁺ ions) is reduced to form hydrogen gas. This marks the negative, or cathodic, edge of the window. The reaction changes with pH:
- In acid:
2H⁺ + 2e⁻ → H₂ - In neutral/alkaline:
2H₂O + 2e⁻ → H₂ + 2OH⁻
How pH Dictates the Potential Range
The dependence of OER and HER on H⁺ and OH⁻ concentration is why the stable window for a glassy carbon electrode shifts so predictably with pH.
Acidic Solutions (e.g., pH 1)
The typical range is +1.3V to -0.9V. A high concentration of H⁺ ions makes it easier to produce hydrogen gas (HER occurs at a less negative potential), shrinking the window on the cathodic side.
Neutral Solutions (pH 7)
The range becomes +0.9V to -1.1V. This represents a baseline where the driving forces for both OER and HER are more balanced.
Alkaline Solutions (e.g., pH 13)
The range shifts to +0.7V to -1.5V. A high concentration of OH⁻ ions makes it easier to produce oxygen gas (OER occurs at a less positive potential), shrinking the window on the anodic side.
Understanding the Practical Trade-offs
The theoretical limits are a guide, but practical work requires additional context.
The Role of Overpotential
Glassy carbon is a popular electrode material precisely because it is a poor catalyst for both OER and HER. This poor catalytic activity, known as high overpotential, requires applying extra voltage beyond the theoretical limit to get the reactions started. This is what gives GCE a wider practical working window than a more catalytic material like platinum.
Exceeding the Potential Window
Applying a potential outside the stable window has two main consequences. First, the massive current from water splitting will completely obscure the electrochemical signal from your analyte. Second, extreme potentials and vigorous gas evolution can physically and chemically damage the electrode surface, leading to unreliable results.
Setting the Right Potential for Your Experiment
Use these ranges as a starting point for designing your electrochemical measurements.
- If your primary focus is on oxidation in acidic media: You have a wide window to work with, up to about +1.3V.
- If your primary focus is on reduction in alkaline media: You can explore very negative potentials, down to approximately -1.5V.
- If you are working in a new electrolyte system: Always run a background scan using only the supporting electrolyte first. This will experimentally reveal the precise working window for your specific conditions before you introduce your analyte.
By understanding that the potential window is defined by the stability of your solvent, you can confidently set the parameters for clean, accurate, and repeatable electrochemical experiments.
Summary Table:
| pH Condition | Anodic Limit (OER) | Cathodic Limit (HER) | Typical Range (vs. Ref.) |
|---|---|---|---|
| Acidic (e.g., pH 1) | +1.3 V | -0.9 V | +1.3 V to -0.9 V |
| Neutral (pH 7) | +0.9 V | -1.1 V | +0.9 V to -1.1 V |
| Alkaline (e.g., pH 13) | +0.7 V | -1.5 V | +0.7 V to -1.5 V |
Achieve precise and reliable results in your lab. Understanding the electrochemical window is critical for successful experiments. KINTEK specializes in high-quality glassy carbon electrodes and lab equipment designed for accuracy and durability. Let our experts help you select the right tools for your specific electrolyte conditions.
Contact KINTALK today to discuss your laboratory needs and enhance your electrochemical workflows!
Visual Guide
Related Products
- Glassy Carbon Electrochemical Electrode
- Glassy Carbon Sheet RVC for Electrochemical Experiments
- Gold Disc Electrode
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Metal Disc Electrode Electrochemical Electrode
People Also Ask
- How to make a glassy carbon electrode? A Guide to the Industrial Pyrolysis Process
- What is a glassy carbon electrode made of? The Engineered Material Powering Electrochemical Analysis
- What are the pre-treatment steps for a glassy carbon electrode before use? Ensure Reliable Electrochemical Data
- What is the proper post-treatment and storage procedure for a glassy carbon electrode? Ensure Reliable, Reproducible Results
- Why is glassy carbon selected for mediator-assisted indirect oxidation of glycerol? The Key to Unbiased Research



















