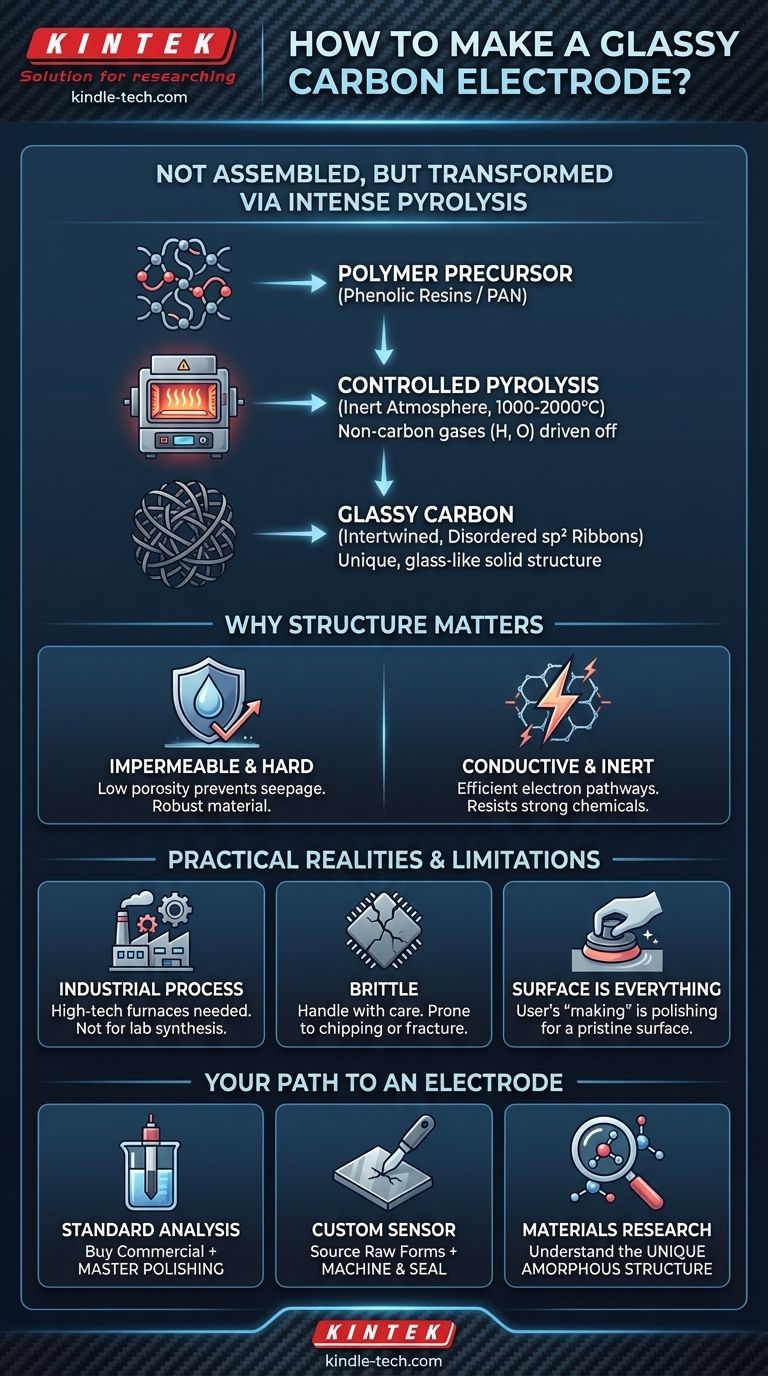
In essence, a glassy carbon electrode is not assembled from parts but is created by transforming a specific type of polymer through intense, controlled heating in an oxygen-free environment. This process, known as pyrolysis, bakes the polymer at temperatures up to 2000 °C, breaking it down and rearranging its carbon atoms into a unique, glass-like solid.
The critical takeaway is that glassy carbon's value comes from its manufacturing process. This controlled pyrolysis creates a disordered, intertwined ribbon structure that is fundamentally different from ordered graphite, giving the material its signature combination of high conductivity, chemical inertness, and impermeability.
The Transformation: From Polymer to Glassy Carbon
The creation of glassy carbon is a feat of materials science, involving a carefully staged thermal decomposition process. It is not something that can be replicated outside of a specialized industrial facility.
The Starting Point: A Polymer Precursor
The process begins not with carbon, but with a highly cross-linked organic polymer. Phenolic resins (like Bakelite) or polyacrylonitrile (PAN) are common starting materials chosen for their ability to form a stable carbon structure upon heating without melting.
The Key Step: Controlled Pyrolysis
The polymer precursor is placed in an inert atmosphere (like nitrogen or argon) and subjected to a slow, meticulously controlled heating schedule. The temperature is gradually raised, often to between 1000 °C and 2000 °C.
This high-temperature baking, or pyrolysis, drives off all non-carbon atoms (like hydrogen and oxygen) as volatile gases. The remaining carbon atoms rearrange to form a new, stable solid.
The Result: An Intertwined, Disordered Structure
Unlike the neat, stacked layers of graphite, the carbon atoms in glassy carbon form a tangled, amorphous structure. It is composed of intertwined ribbons of sp²-hybridized carbon, similar to fragments of graphene sheets, but without any long-range crystalline order. This "frozen" disordered state is what gives it a glass-like appearance and name.
Why This Structure Matters
The unique structure born from pyrolysis directly translates to the properties that make glassy carbon so valuable in electrochemistry and other fields.
Exceptional Hardness and Impermeability
The tangled network of strong carbon-carbon bonds results in a very hard material. More importantly, this structure has extremely low porosity, making it effectively impermeable to gases and liquids. This prevents analytes or solvents from seeping into the electrode body, ensuring that electrochemical reactions only occur at the polished surface.
High Conductivity and Chemical Inertness
Despite being disordered, the extensive network of sp²-hybridized carbon provides excellent pathways for electrons to travel, resulting in high electrical conductivity. The stable, all-carbon structure is also exceptionally chemically inert, resisting attack from strong acids, bases, and aggressive organic solvents.
Understanding the Practical Realities
While the manufacturing process is fascinating, it comes with practical limitations that are critical to understand.
This is an Industrial Process
Creating glassy carbon requires specialized furnaces, precise atmospheric and temperature control, and significant energy input. It is a high-tech manufacturing process, not a laboratory synthesis. For researchers and engineers, "making" an electrode almost always means purchasing the material from a commercial supplier.
Brittleness is a Key Weakness
While very hard, glassy carbon is also brittle. It can easily chip or fracture if dropped or subjected to mechanical shock. This is a primary cause of electrode failure.
Surface Preparation is Everything
The performance of a glassy carbon electrode is dictated almost entirely by the condition of its surface. The manufacturing process creates the bulk material, but the user is responsible for "making" the functional surface through careful polishing and cleaning before each use. An unpolished or contaminated surface will yield poor, unreliable, and irreproducible results.
How to "Make" an Electrode for Your Goal
For virtually all users, the practical task is not manufacturing the material itself, but preparing a commercial product for a specific application.
- If your primary focus is standard electroanalysis: Purchase a high-quality commercial glassy carbon electrode. Your "making" process will be mastering the art of mechanical and electrochemical polishing to create a pristine, repeatable surface for your measurements.
- If your primary focus is fabricating a custom sensor: You will need to source glassy carbon in raw forms, such as plates or rods, from a specialized materials supplier. Your work will then involve machining, cutting, and sealing this material into your desired device architecture.
- If your primary focus is materials research: Understand that "glassy carbon" is a specific class of material defined by its amorphous structure and polymer precursor, setting it apart from other carbon forms like pyrolytic graphite, diamond, or carbon fibers.
Ultimately, your success with a glassy carbon electrode depends on preparing its surface, not its bulk.

Summary Table:
| Key Manufacturing Step | Details |
|---|---|
| Precursor Material | Phenolic resin or polyacrylonitrile polymer |
| Process | Pyrolysis in inert atmosphere (nitrogen/argon) |
| Temperature Range | 1000°C to 2000°C |
| Resulting Structure | Amorphous, intertwined ribbons of sp² carbon |
| Key Properties | High conductivity, chemical inertness, impermeability |
Ready to advance your electrochemical research with high-quality materials? KINTEK specializes in providing the lab equipment and consumables you need for cutting-edge electrochemistry. Whether you're working with glassy carbon electrodes or other specialized materials, our expertise ensures you have the right tools for success. Contact us today to discuss how we can support your laboratory's specific requirements!
Visual Guide
Related Products
- Glassy Carbon Electrochemical Electrode
- Glassy Carbon Sheet RVC for Electrochemical Experiments
- Gold Disc Electrode
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Reference Electrode Calomel Silver Chloride Mercury Sulfate for Laboratory Use
People Also Ask
- What are the properties of graphite rods? Leverage High Conductivity for Extreme Applications
- How is the working electrode sample made conductive? Using Copper Tape for Reliable Electrochemical Analysis
- What is the role of the near-zero gap structure in a Membrane Electrode Assembly (MEA)? Enhance Efficiency Now
- What are the application areas for the Platinum-Titanium Functional Electrode? A Guide to High-Performance Electrochemical Solutions
- What are the key precautions for a gold disc electrode? Ensure Accurate Results & Long Lifespan
- When to use a platinum electrode? Ensure Reliable and Accurate Electrochemical Results
- How should a portable copper sulfate reference electrode be used during an experiment? Ensure Accurate Electrochemical Measurements
- Why are titanium rods used in MEC electrode construction? Ensure High Conductivity and Corrosion Resistance



















