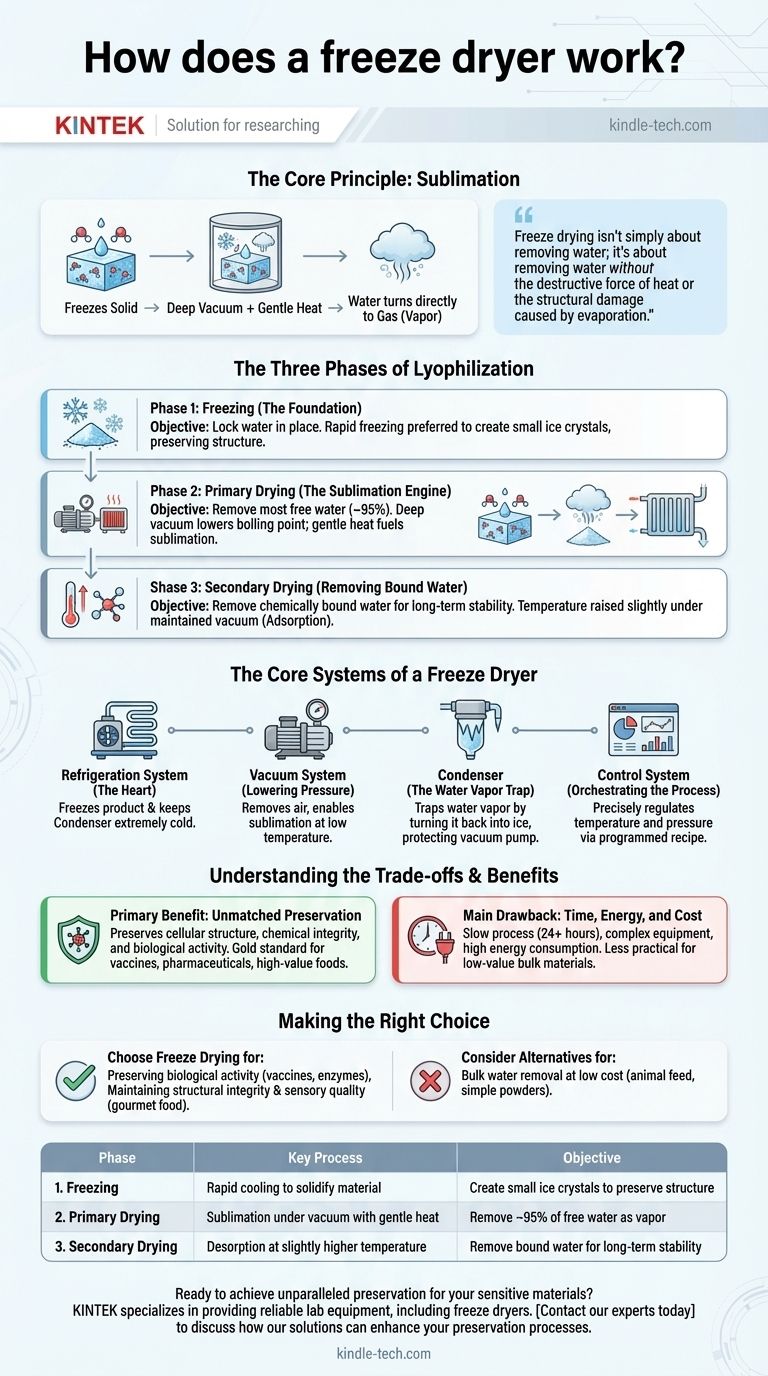
At its core, a freeze dryer works by leveraging a physical process called sublimation. It first freezes a material solid, then places it under a deep vacuum. By carefully adding a small amount of heat, the frozen water turns directly into a gas (water vapor) without ever melting into a liquid, preserving the material's delicate structure and chemical composition.
The crucial insight is that freeze drying isn't simply about removing water; it's about removing water without the destructive force of heat or the structural damage caused by evaporation. This low-temperature, low-pressure process is the key to preserving the integrity of sensitive materials, from pharmaceuticals to high-value foods.

The Three Phases of Lyophilization
The entire freeze-drying process, formally known as lyophilization, is a precisely controlled procedure broken down into three distinct phases. Each phase has a specific objective in achieving a perfectly preserved, shelf-stable product.
Phase 1: Freezing (The Foundation)
Before any drying can occur, the product must be completely frozen solid. The way a product is frozen directly impacts the quality of the final result.
Rapid freezing is often preferred because it creates small ice crystals. Large, slow-forming ice crystals can puncture cell walls and damage the material's microscopic structure. The goal is to lock the water in place while causing minimal disruption.
Phase 2: Primary Drying (The Sublimation Engine)
This is the longest and most critical phase, where most of the water is removed. Two conditions are created simultaneously: a deep vacuum and a controlled input of heat.
The vacuum dramatically lowers the pressure inside the drying chamber. This reduces the boiling point of water so significantly that the frozen ice can transition directly into water vapor—the process of sublimation.
Gentle heat is applied to the shelves holding the product. This heat does not melt the ice but instead provides the necessary energy to fuel the sublimation process. As the ice sublimates, a "sublimation front" of dried material is left behind.
Phase 3: Secondary Drying (Removing Bound Water)
After primary drying, a small amount of moisture remains, chemically bound to the molecules of the material itself. This water cannot be sublimated.
To remove it, the temperature is raised slightly while the vacuum is maintained. This process, known as adsorption, breaks the bonds between the water molecules and the material, allowing the final traces of moisture to be drawn away. This step is critical for achieving long-term stability.
The Core Systems of a Freeze Dryer
Four interdependent systems work in concert to create the precise conditions required for lyophilization. Understanding their roles is key to understanding the machine itself.
The Refrigeration System: Creating the Cold
Often called the "heart" of the freeze dryer, this system has two jobs. First, it freezes the product during the initial phase. Second, and just as important, it keeps the condenser (or cold trap) at an extremely low temperature throughout the drying process.
The Vacuum System: Lowering the Pressure
A powerful vacuum pump is responsible for removing air from the drying chamber. This creates the low-pressure environment that is absolutely essential for sublimation to occur at a low temperature. Without the vacuum, the ice would simply melt.
The Condenser: The Critical Water Vapor Trap
As water vapor sublimates from the product, it needs somewhere to go. The condenser is an array of extremely cold coils or plates located between the drying chamber and the vacuum pump.
This surface is much colder than the product itself. As the water vapor travels toward the vacuum pump, it hits the condenser and instantly turns back into ice, effectively trapping it. This prevents water vapor from entering and damaging the vacuum pump.
The Control System: Orchestrating the Process
Modern freeze dryers use a sophisticated control system to manage the entire process. It precisely regulates the shelf temperature and chamber pressure according to a programmed "recipe," ensuring each of the three phases is executed perfectly for a specific product.
Understanding the Trade-offs and Benefits
While incredibly effective, freeze drying is not the right solution for every situation. Its advantages come with specific costs.
The Primary Benefit: Unmatched Preservation
By avoiding the liquid phase and high temperatures, freeze drying preserves cellular structure, chemical integrity, and biological activity. This is why it is the gold standard for sensitive materials like vaccines, antibodies, enzymes, and pharmaceuticals. For food, it preserves color, flavor, and nutrition far better than any other drying method.
The Main Drawback: Time, Energy, and Cost
The freeze-drying process is slow, often taking 24 hours or more to complete a single batch. The equipment is complex and expensive, and the combination of deep refrigeration and vacuum pumping consumes a significant amount of energy. This makes it less practical for low-value, bulk materials.
Making the Right Choice for Your Goal
Deciding whether to use freeze drying depends entirely on the value you place on preservation.
- If your primary focus is preserving biological activity: For vaccines, enzymes, or microbial cultures, freeze drying is the essential and non-negotiable choice.
- If your primary focus is maintaining structural integrity and sensory quality: For gourmet coffee, astronaut food, or high-end fruit, freeze drying provides a superior result that justifies the cost.
- If your primary focus is simply bulk water removal at low cost: For animal feed or simple industrial powders, conventional heat drying or spray drying are far more economical and efficient methods.
Ultimately, choosing freeze drying is a decision to invest in unparalleled preservation over speed and low cost.
Summary Table:
| Phase | Key Process | Objective |
|---|---|---|
| 1. Freezing | Rapid cooling to solidify the material | Create small ice crystals to preserve structure |
| 2. Primary Drying | Sublimation under vacuum with gentle heat | Remove ~95% of free water as vapor |
| 3. Secondary Drying | Desorption at slightly higher temperature | Remove bound water for long-term stability |
Ready to achieve unparalleled preservation for your sensitive materials?
Freeze drying is the gold standard for maintaining the integrity of pharmaceuticals, high-value foods, and biological samples. KINTEK specializes in providing reliable lab equipment, including freeze dryers, to meet the precise needs of your laboratory.
Contact our experts today to discuss how our solutions can enhance your preservation processes and ensure the highest quality results for your critical applications.
Visual Guide
Related Products
- Benchtop Laboratory Freeze Dryer for Lab Use
- Benchtop Laboratory Vacuum Freeze Dryer
- Laboratory Sterilizer Lab Autoclave Pulse Vacuum Lifting Sterilizer
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
- Laboratory Test Sieves and Sieving Machines
People Also Ask
- What is the function of Freeze-thaw Equipment in Au-(PNiPAAm/PVA) hydrogel? Achieve High-Speed Photothermal Actuation
- Why is a laboratory freeze-drying system essential for fermentation biomass? Preserve Sample Integrity for Analysis
- Why is a laboratory vacuum freeze dryer essential for plant extracts? Preserve Bioactivity & Structure
- What is the function of a freeze dryer in the ice-templating process? Preserving Aligned Pore Scaffolds for LAGP
- Why is a freeze dryer preferred for reduced graphene oxide (Hh-RGO) powders? Preserve Nano-Structure and Performance



















