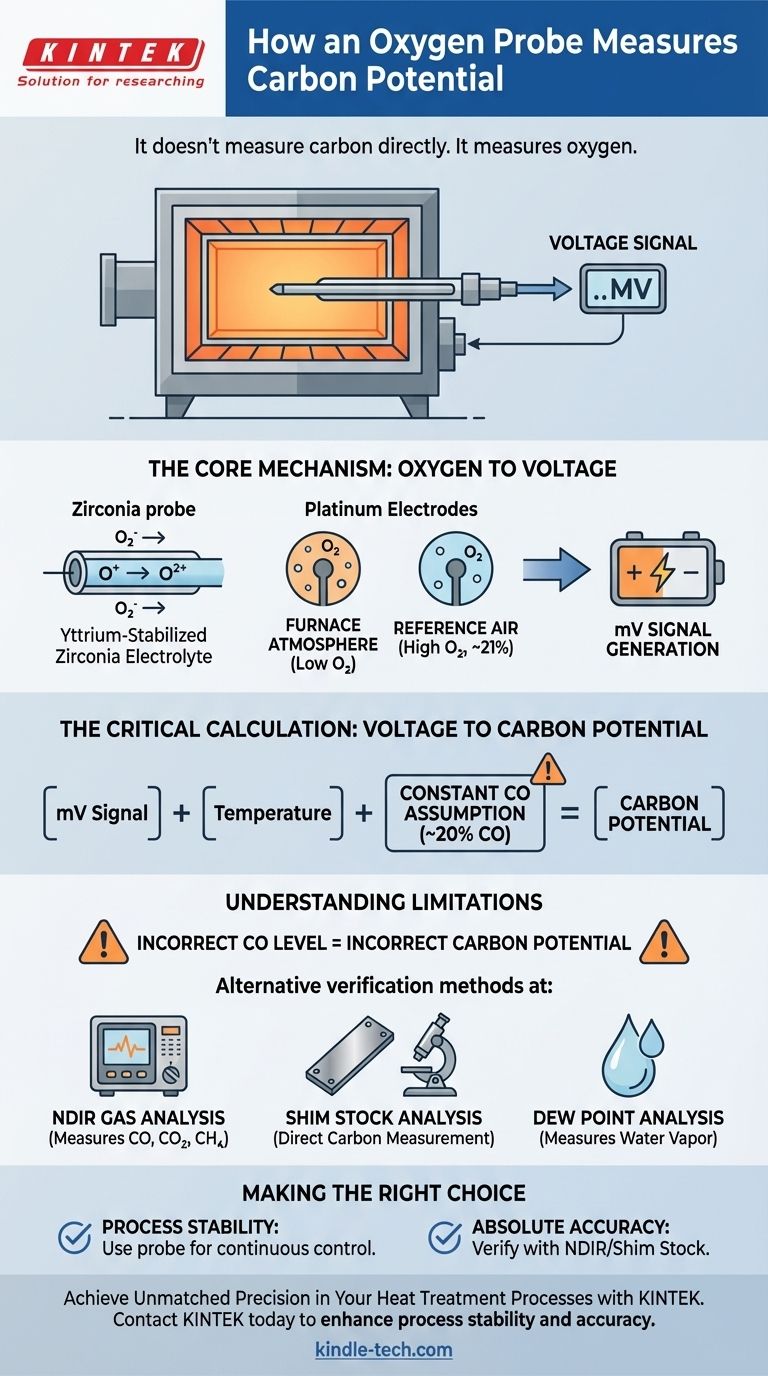
At its core, a carbon probe does not measure carbon directly. Instead, it measures the partial pressure of oxygen within the furnace atmosphere. This oxygen measurement is then used in a calculation to determine the carbon potential, based on a critical assumption about the furnace's gas composition.
The probe functions as a tiny oxygen-powered battery. It generates a small voltage based on the difference in oxygen levels between a known reference air source and the furnace atmosphere, which is then mathematically converted into a carbon potential reading.

The Core Mechanism: From Oxygen to Voltage
An oxygen or carbon probe is an electrochemical sensor, often referred to as a zirconia probe. Its operation relies on fundamental principles of chemistry and materials science to provide a continuous, real-time signal from within the harsh furnace environment.
The Zirconia Electrolyte
The heart of the probe is a closed-end tube made of yttrium-stabilized zirconia. This ceramic material has a unique property: at high temperatures, it becomes an electrolyte, allowing oxygen ions to pass through it.
The Platinum Electrodes
Two platinum electrodes are coated on the zirconia tube—one on the inside and one on the outside. The outer electrode is exposed to the furnace atmosphere, while the inner electrode is supplied with a constant flow of reference air, which has a known oxygen concentration (approximately 21%).
Generating the Signal
At operating temperature, the vast difference in oxygen partial pressure between the furnace atmosphere (very low oxygen) and the reference air (high oxygen) causes oxygen ions to move through the zirconia electrolyte. This movement of ions creates a measurable DC millivolt signal between the two platinum electrodes.
The Critical Calculation: Translating Voltage to Carbon
The raw millivolt signal from the probe is directly proportional to the oxygen level, but it is not the final carbon potential value. That conversion requires a crucial calculation that rests on a key assumption about the furnace atmosphere.
The Role of Carbon Monoxide (CO)
The calculation that converts the probe's voltage to carbon potential assumes that the concentration of carbon monoxide (CO) in the furnace atmosphere is stable and constant, typically around 20%.
The Chemical Equilibrium
In a carburizing atmosphere, the gases (CO, CO2, and O2) are in a state of equilibrium with the dissolved carbon in the steel. By measuring the minute amount of oxygen, and assuming the CO level is fixed, the controller can accurately infer the balance between CO and CO2, which directly dictates the carbon potential of the atmosphere.
Understanding the Limitations
While oxygen probes are an industry standard due to their durability and quick response, their accuracy is entirely dependent on the validity of their core operating assumption.
The Constant CO Assumption
If the carbon monoxide (CO) level deviates significantly from the assumed 20%, the calculated carbon potential will be incorrect, even if the probe is functioning perfectly. This can happen if the endothermic generator is malfunctioning or if there are air leaks in the furnace.
The Need for Verification
Because the probe doesn't measure carbon directly, its readings should be periodically verified. This ensures the entire system—from gas generation to the final calculation—is producing an accurate result for the specific steel and temperature being used.
Alternative Verification Methods
Several methods exist to validate the probe's readings or provide a direct measurement of the atmosphere's properties. These include:
- NDIR (Non-Dispersive Infrared) Gas Analysis: Measures CO, CO2, and CH4 concentrations directly.
- Shim Stock Analysis: A small piece of steel foil is processed and its carbon content is measured directly.
- Dew Point Analysis: Measures the water vapor content, which is another way to determine the oxygen partial pressure.
Making the Right Choice for Your Goal
Understanding how an oxygen probe works is key to using it effectively for precise atmosphere control.
- If your primary focus is process stability: Rely on the continuous, real-time feedback from the oxygen probe to maintain a consistent furnace atmosphere, but be aware that its accuracy hinges on a stable CO level.
- If your primary focus is absolute accuracy: Use the oxygen probe for minute-to-minute control, but implement a regular schedule of verification using a secondary method like NDIR or shim stock analysis to confirm its readings are correct.
Ultimately, the oxygen probe is a powerful tool for process control when its principles, and its limitations, are fully understood.
Summary Table:
| Key Component | Function |
|---|---|
| Zirconia Electrolyte | Allows oxygen ions to pass through at high temperatures. |
| Platinum Electrodes | Generate a millivolt signal based on oxygen difference. |
| Reference Air | Provides a known oxygen level (21%) for comparison. |
| Constant CO Assumption | Critical for converting oxygen reading to carbon potential (typically 20%). |
Achieve Unmatched Precision in Your Heat Treatment Processes
Understanding the science behind your furnace's atmosphere is the first step to superior results. KINTEK specializes in high-performance lab equipment, including the robust sensors and analyzers needed to monitor and control your furnace atmosphere with confidence.
Whether you rely on oxygen probes for real-time control or need secondary verification methods like NDIR gas analysis, we provide the reliable tools and expert support for your laboratory's success.
Contact KINTEK today to discuss your specific furnace control challenges and discover how our solutions can enhance your process stability and accuracy.
Visual Guide
Related Products
- Sub-Lance Probe for Molten Steel Temperature Carbon Content Oxygen Content Measurement and Steel Sample Collection
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- Battery Lab Equipment Battery Capacity and Comprehensive Tester
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
People Also Ask
- What is the function of a tubular atmosphere furnace? Optimize Al/SiC Annealing at 700°C with Inert Environments
- What does the inert gas do in the process? Ensure Material Integrity with Non-Reactive Control
- What is hydrogen annealing? Achieve Superior Material Properties with Bright Annealing
- Why is a high-temperature furnace with atmosphere control required for rGO? Enhance Your Carbon Research Quality
- How does a hydrogen furnace work? Master High-Purity, Oxide-Free Heat Treatment
- What is atmosphere controlled furnace? Prevent Oxidation and Enable Advanced Material Processing
- Which gases prevent oxidation? A Guide to Inert and Reducing Atmospheres
- What is the difference between oxidizing and reducing atmospheres? Key Insights for Your Applications



















