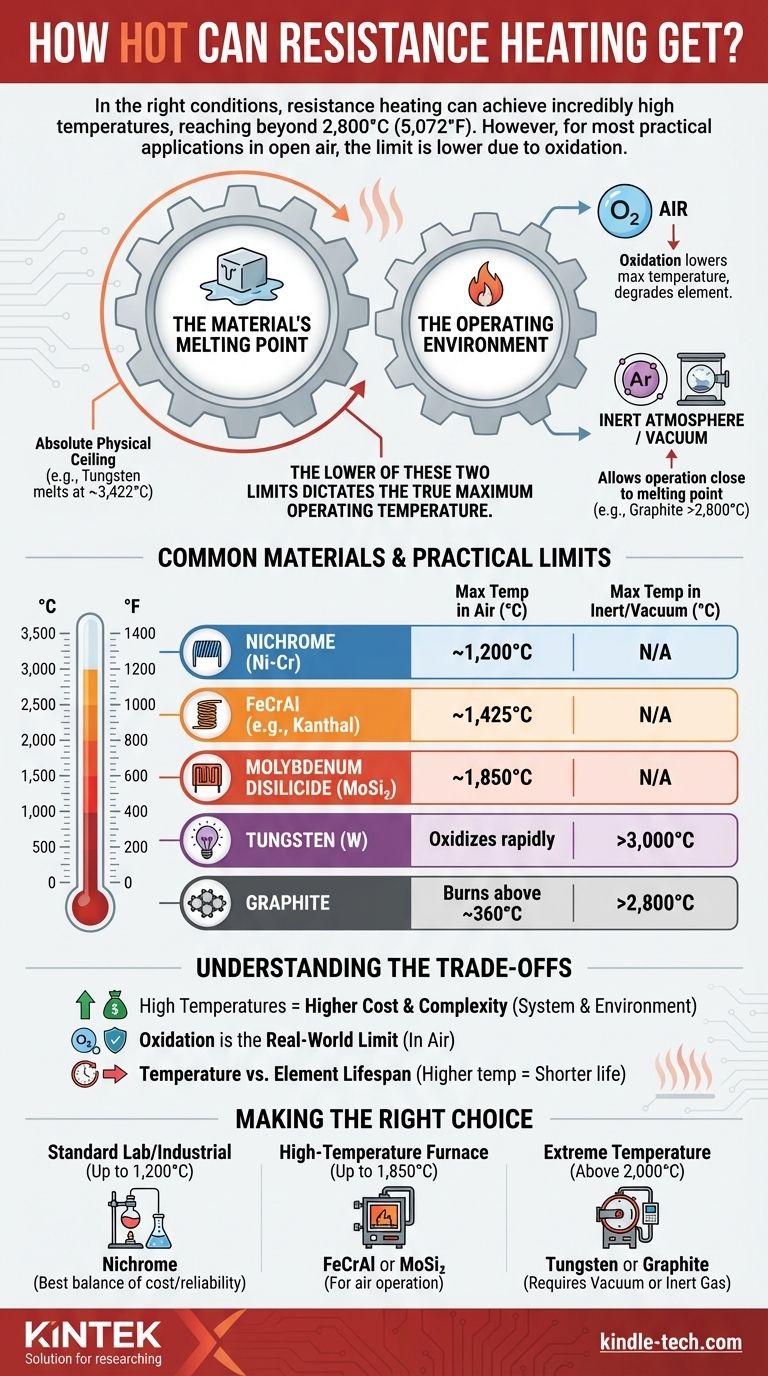
In the right conditions, resistance heating can achieve incredibly high temperatures, reaching beyond 2,800°C (5,072°F). However, for most practical applications operating in open air, the maximum temperature is significantly lower, often limited by the material's reaction with oxygen long before it reaches its melting point.
The maximum temperature of a resistance heater is not one single value. It is a direct result of two competing factors: the physical melting point of the heating element and the chemical degradation of that element in its operating environment.

The Two Factors Defining Maximum Temperature
To understand the limits of resistance heating, you must consider both the material itself and the atmosphere surrounding it. The lower of these two limits will always dictate the true maximum operating temperature.
The Material's Melting Point
The absolute physical ceiling for any resistance heater is the temperature at which the element material itself melts or, in some cases, sublimes (turns directly into a gas).
This is why material selection is the first critical decision. Different materials have vastly different melting points. For example, tungsten melts at 3,422°C (6,192°F), while common nickel-chromium alloys melt closer to 1,400°C (2,550°F).
The Operating Environment: Air vs. Inert Atmosphere
This is the most important practical consideration. The presence of oxygen in the air dramatically lowers the effective maximum temperature for most materials.
At high temperatures, the element material will begin to oxidize, or chemically react with the oxygen in the air. This process degrades the element, causing it to fail far below its melting point.
In an inert atmosphere (like argon gas) or a vacuum, there is no oxygen to cause this degradation. This allows the heating element to operate at temperatures much closer to its true melting point. This is why a graphite element, which begins to burn away in air above 360°C, can be used to achieve over 2,800°C in an inert environment.
Common Materials and Their Practical Limits
The choice of material is a direct trade-off between cost, durability, and maximum operating temperature in a given environment.
Nickel-Chromium (Nichrome) Alloys
Nichrome is the workhorse of resistance heating for general-purpose applications. It forms a protective outer layer of chromium oxide that prevents further oxidation, allowing it to operate reliably in air up to about 1,200°C (2,190°F).
Iron-Chromium-Aluminum (FeCrAl) Alloys
Often known by the brand name Kanthal, these alloys are a step up from Nichrome. They form a resilient aluminum oxide layer that allows for higher operating temperatures in air, typically up to 1,425°C (2,600°F).
Molybdenum Disilicide (MoSi₂)
For very high-temperature industrial furnaces operating in air, MoSi₂ elements are used. These can operate continuously at temperatures up to 1,850°C (3,360°F).
Refractory Metals (Tungsten & Molybdenum)
These materials have extremely high melting points but oxidize almost instantly in air at high temperatures. They are reserved exclusively for vacuum or inert gas furnaces, where tungsten can safely exceed 3,000°C (5,432°F).
Graphite
Like refractory metals, graphite is only suitable for oxygen-free environments. In a vacuum or inert gas, it can reach temperatures well above 2,800°C (5,072°F), making it a common choice for extreme-temperature furnaces.
Understanding the Trade-offs
Selecting a heating solution is rarely about achieving the absolute maximum temperature. It's about finding the right balance for your specific needs.
The Cost of High Temperatures
As you move up the temperature scale, the cost and complexity of the system increase exponentially. High-temperature materials are more expensive, and the requirement for a vacuum or inert gas environment adds significant cost and engineering challenges.
Oxidation is the Real-World Limit
For any application that operates in open air, the material's resistance to oxidation—not its melting point—is the defining limitation. Pushing an element past its recommended temperature in air will drastically shorten its lifespan.
Temperature vs. Element Lifespan
Even within the recommended range, there is a trade-off between operating temperature and lifespan. An element run continuously at its maximum rated temperature will fail much sooner than one run at 100 degrees cooler.
Making the Right Choice for Your Application
The ideal resistance heating material is determined entirely by your target temperature and operating environment.
- If your primary focus is standard industrial or lab heating in air (up to 1,200°C): Nichrome alloys provide the best balance of cost and reliability.
- If your primary focus is high-temperature furnace work in air (up to 1,850°C): FeCrAl alloys or, for the highest temperatures, Molybdenum Disilicide (MoSi₂) elements are required.
- If your primary focus is extreme temperature processing (above 2,000°C): You must use a vacuum or inert gas furnace with refractory metal (like Tungsten) or Graphite elements.
By understanding the interplay between material and environment, you can select a resistance heating solution that is both effective and durable for your goal.
Summary Table:
| Material | Max Temp in Air (°C) | Max Temp in Inert/Vacuum (°C) | Common Applications |
|---|---|---|---|
| Nichrome (Ni-Cr) | ~1,200°C | N/A | General-purpose industrial/lab heating |
| FeCrAl (e.g., Kanthal) | ~1,425°C | N/A | High-temperature furnaces |
| Molybdenum Disilicide (MoSi₂) | ~1,850°C | N/A | Industrial high-temp furnaces |
| Tungsten (W) | Oxidizes rapidly | >3,000°C | Extreme-temperature vacuum/inert furnaces |
| Graphite | Burns above ~360°C | >2,800°C | High-temperature processing furnaces |
Need a Reliable Heating Solution for Your Lab?
Choosing the right heating element is critical for your process's success and equipment longevity. The experts at KINTEK understand the precise balance between temperature, environment, and material durability.
We specialize in supplying high-quality lab equipment and consumables, including robust resistance heating solutions tailored to your specific application—whether you require standard heating in air or extreme temperatures in a controlled atmosphere.
Let us help you achieve precise and consistent results. Contact our technical team today to discuss your requirements and find the optimal heating solution for your laboratory needs.
Visual Guide
Related Products
- Molybdenum Disilicide (MoSi2) Thermal Elements Electric Furnace Heating Element
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
- Cylindrical Lab Electric Heating Press Mold for Laboratory Applications
- Thermally Evaporated Tungsten Wire for High Temperature Applications
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
People Also Ask
- Why does the temperature of the heating element increase? To Drive Efficient Heat Transfer
- How do PTC fan heaters work? Discover Efficient Space Heating Solutions for Labs & Offices
- What are the key properties of metallic heating elements in heat treatment furnaces?
- What is the operating principle of a resistance wire heater? Insights into Joule Heating and Precise Thermal Control
- What function do molybdenum disilicide heating elements perform? Precision Heat for Pulverized Coal Research
- Is graphite used as a refractory material? Discover Its Unmatched High-Temperature Performance
- What is special about tungsten? The Ultimate Metal for Extreme Heat & Wear Resistance
- What is a thermocouple and how does it function in a sintering furnace? Master Precise High-Temp Control



















