To properly handle a gold plate electrode during an experiment, you must secure it correctly within your apparatus, ensuring that only the gold surface itself makes contact with the electrolyte solution. It is critical to operate within the electrode's specified voltage and current limits while shielding it from electromagnetic interference. These steps are fundamental to protecting both the integrity of your electrode and the validity of your data.
The reliability of your electrochemical experiment hinges on protecting the electrode's physical integrity and controlling its environment. Your primary goal is to isolate the reaction at the gold surface from all other variables, including mechanical stress, contamination from the electrode holder, and electrical noise.

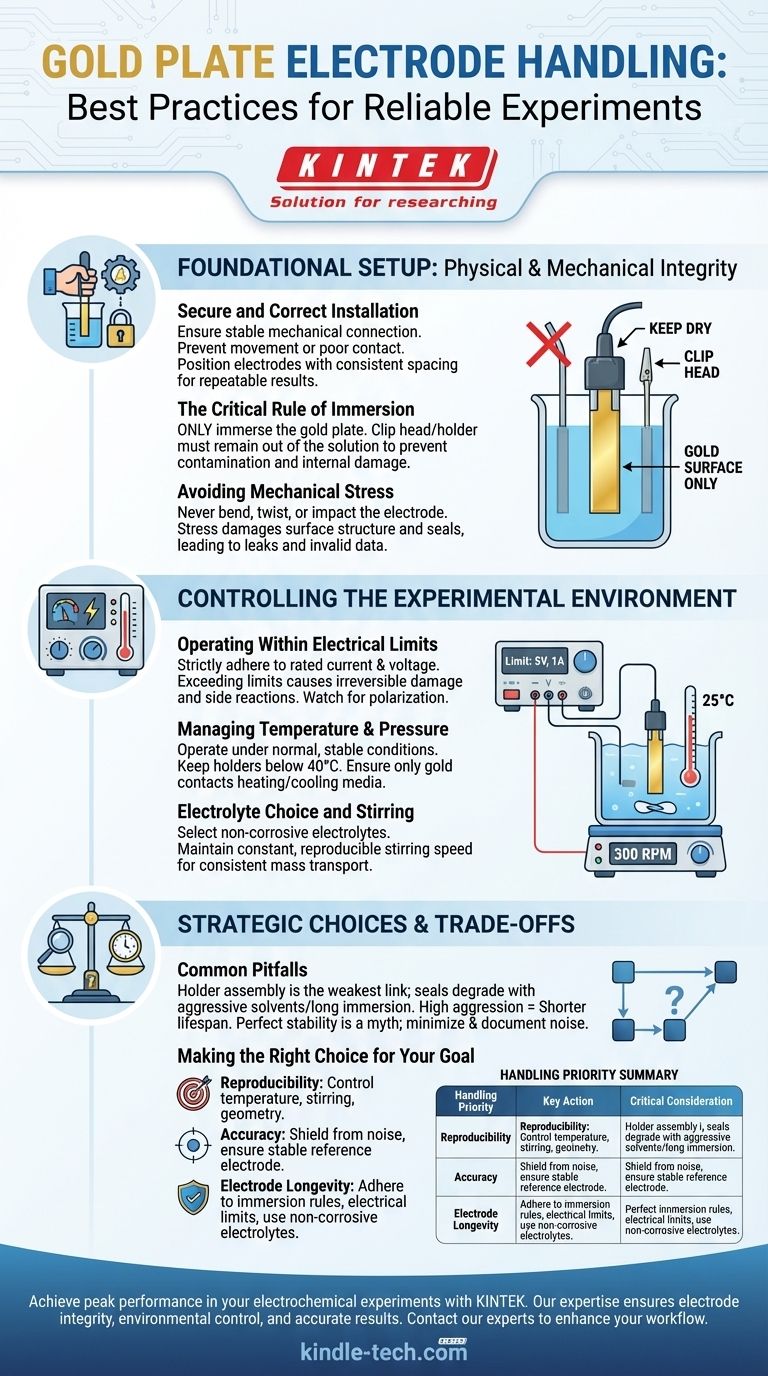
Foundational Setup: Physical and Mechanical Integrity
The most common errors occur during the initial physical setup. A flawed setup guarantees flawed data and risks permanent damage to the electrode.
Ensuring Secure and Correct Installation
A stable mechanical connection is non-negotiable. The electrode must be installed firmly in the apparatus to prevent poor electrical contact or movement during measurement.
Position the gold plate electrode with appropriate and consistent spacing relative to the reference and counter electrodes. This geometry directly impacts the electric field and mass transport, making consistency crucial for repeatable results.
The Critical Rule of Immersion
For electrodes where a gold plate is held by a clip or holder, only the gold plate itself should be immersed in the electrolyte.
The clip head or holder must remain completely out of the solution. These components often contain solder points sealed with adhesive, which can degrade upon immersion, contaminating your electrolyte and allowing liquid to damage the electrode's internal connections.
Avoiding Mechanical Stress
Never subject the electrode to bending, twisting, impact, or excessive pressure.
Mechanical stress can alter the microscopic surface structure of the gold plate or damage the seal between the plate and its insulating body. This changes the electrochemically active surface area and can create pathways for leaks, invalidating your results.
Controlling the Experimental Environment
Your electrode does not operate in a vacuum. The chemical and physical environment dictates its performance and lifespan.
Operating Within Electrical Limits
Strictly adhere to the rated current and voltage limits specified for your electrode. Exceeding these parameters can induce unwanted side reactions, cause irreversible surface damage, or destroy the electrode.
Be aware of polarization effects during current measurements. High currents can alter the surface and the local electrolyte composition, skewing potential readings.
Managing Temperature and Pressure
It is almost always best to conduct experiments under normal, stable temperature and pressure. Many electrode holders are not designed to exceed 40°C.
If temperature control is required using a water bath or heater, ensure that only the gold portion of the electrode contacts the heating or cooling medium. The main body and connector must be kept separate.
Electrolyte Choice and Stirring
Select an electrolyte that is non-corrosive to both the gold plate and any materials used in the electrode holder.
If your experiment requires stirring, maintain a constant and reproducible stirring speed. This ensures consistent mass transport of reactants to the electrode surface, which is critical for many quantitative electrochemical methods.
Understanding the Trade-offs and Common Pitfalls
Perfect experimental conditions are an ideal, but awareness of practical limitations is what separates a novice from an expert.
The Holder Is Often the Weakest Link
The single most common point of failure is not the gold plate itself, but the holder assembly. The seal between the conductive element and the insulating body is a vulnerability. Aggressive solvents or prolonged immersion can compromise this seal, leading to contamination and equipment failure.
Aggressive Conditions vs. Electrode Lifespan
Your experiment may require high temperatures, extreme pH levels, or high potentials. Understand that these conditions will drastically shorten the electrode's lifespan. There is a direct trade-off between the aggressiveness of your experiment and the longevity of your equipment.
The Myth of a "Perfectly" Stable System
External factors like electromagnetic fields from other lab equipment and subtle mechanical vibrations can introduce noise into your data. While shielding and grounding are crucial, the practical goal is to minimize and document these variables rather than assume they can be eliminated entirely.
Making the Right Choice for Your Goal
Your specific handling protocol should align with your experimental priorities. Use these principles to guide your actions.
- If your primary focus is reproducibility: Emphasize precise and consistent control over temperature, stirring speed, and the geometric positioning of all electrodes for every run.
- If your primary focus is accuracy: Prioritize shielding from all electrical noise and ensuring the reference electrode is stable, correctly placed, and functioning properly.
- If your primary focus is electrode longevity: Strictly adhere to the immersion rule, operate well within the electrode's electrical limits, and choose the least corrosive electrolyte that meets your experimental needs.
By treating the entire electrochemical cell as an integrated system, you transform the electrode from a simple component into a reliable instrument for discovery.
Summary Table:
| Handling Priority | Key Action | Critical Consideration |
|---|---|---|
| Reproducibility | Control temperature, stirring, and electrode geometry. | Ensures consistent mass transport and electric field for every run. |
| Accuracy | Shield from electrical noise; ensure stable reference electrode. | Minimizes interference for reliable potential and current measurements. |
| Electrode Longevity | Adhere to immersion rules and electrical limits; use non-corrosive electrolytes. | Protects the seal and gold surface from degradation and damage. |
Achieve peak performance in your electrochemical experiments with KINTEK.
Proper electrode handling is the foundation of reliable data. At KINTEK, we specialize in providing the high-quality lab equipment and consumables your laboratory needs to succeed. Our expertise ensures you have the right tools and support to maintain electrode integrity, control your experimental environment, and obtain accurate, reproducible results.
Let us help you protect your investment and advance your research. Contact our experts today to discuss your specific laboratory requirements and discover how our solutions can enhance your workflow.
Visual Guide
Related Products
- Gold Electrochemical Sheet Electrode Gold Electrode
- Gold Disc Electrode
- Metal Disc Electrode Electrochemical Electrode
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Graphite Disc Rod and Sheet Electrode Electrochemical Graphite Electrode
People Also Ask
- What is the critical rule for using a gold plate electrode? Ensure Only the Gold Surface Contacts the Electrolyte
- What are the disadvantages of gold electrodes? Key Limitations for Your Lab Projects
- What precautions should be taken to prevent mechanical damage to a gold plate electrode? Protect Your Data Integrity
- How should contamination of a gold plate electrode be prevented and managed? Essential Care for Reliable Data
- What are gold electrodes used for? Achieve Unmatched Sensitivity in Biosensing and Research



















