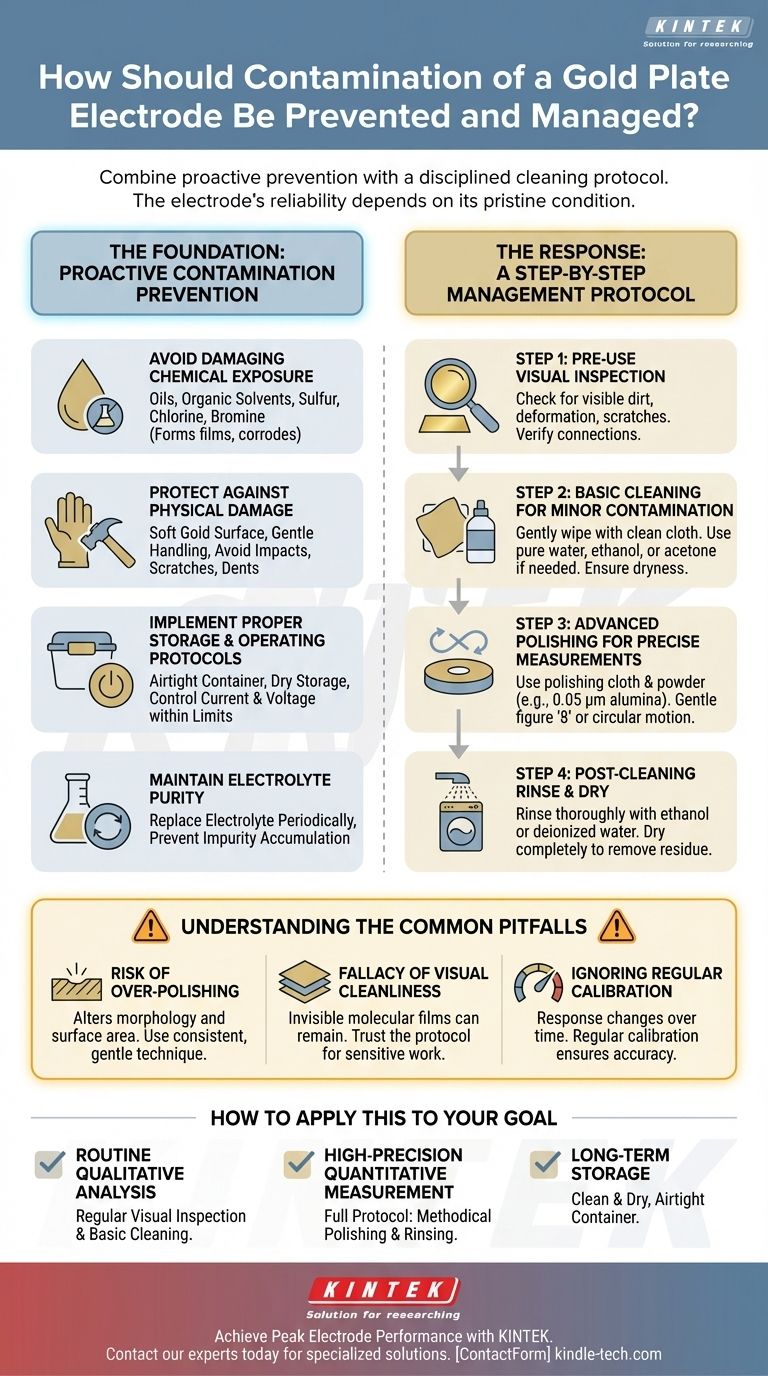
To effectively manage a gold plate electrode, you must combine proactive prevention with a disciplined cleaning protocol. Contamination is best prevented by avoiding contact with oils, organic solvents, and corrosive elements like sulfur and chlorine, while physical damage is avoided by handling the soft gold surface with extreme care. If contamination occurs, it must be addressed immediately through a methodical cleaning process, ranging from a simple solvent wipe to mechanical polishing for high-precision applications.
The core principle is that the electrochemical reliability of a gold electrode is entirely dependent on the pristine condition of its surface. This demands a rigorous, two-part strategy: proactively shielding the electrode from all potential contaminants and methodically restoring its surface integrity before each critical use.

The Foundation: Proactive Contamination Prevention
An effective management strategy begins long before the experiment. Preventing contamination is always more efficient than correcting it.
Avoid Damaging Chemical Exposure
Gold surfaces are highly susceptible to chemical bonding and corrosion. Avoid exposing the electrode to oils and organic solvents, as these can form insulating films on the surface.
Also, keep the electrode away from any substances containing sulfur, chlorine, or bromine, as these elements can chemically react with and corrode the gold surface.
Protect Against Physical Damage
The gold plate itself is soft and malleable, making it extremely vulnerable to physical damage.
Handle the electrode gently at all times. Avoid any impacts, squeezing, or contact with hard objects that could cause scratches, dents, or deformation, all of which alter its electrochemical properties.
Implement Proper Storage and Operating Protocols
When not in use, the electrode should be thoroughly dried and kept in a dedicated, sealed container to protect it from atmospheric contaminants.
During operation, carefully control the applied current and voltage to remain within the electrode's specified tolerance range. Exceeding these limits can damage the surface.
Maintain Electrolyte Purity
The electrolyte is a common source of contamination. To prevent the accumulation of impurities that can deposit onto the electrode, replace the electrolyte periodically based on its usage frequency.
The Response: A Step-by-Step Management Protocol
When cleaning is necessary, a structured approach ensures the surface is restored without causing further damage.
Step 1: Pre-Use Visual Inspection
Before every use, carefully inspect the electrode surface for any visible dirt, deformation, or scratches. Verify that all electrical connections are secure and stable.
Step 2: Basic Cleaning for Minor Contamination
For minor surface dirt, gently wipe the electrode with a clean, soft cloth.
If needed, use pure water, ethanol, or acetone as a cleaning solvent. After wiping, ensure the electrode is completely dry before proceeding.
Step 3: Advanced Polishing for Precise Measurements
For high-precision work, a simple wipe is insufficient. Mechanical polishing is required to create a clean, uniform, and reproducible surface.
Hold the electrode vertically against a polishing cloth moistened with distilled water and a small amount of polishing powder (e.g., 0.05 µm alumina).
Polish the surface using a gentle figure '8' or circular motion. This process removes the top layer of gold, along with any embedded contaminants.
Step 4: Post-Cleaning Rinse and Dry
After polishing, the electrode must be thoroughly cleaned to remove all polishing residue. Rinse it carefully with ethanol or deionized water.
Finally, dry the electrode completely. This step is critical to prevent residual solvent from interfering with your experiment.
Understanding the Common Pitfalls
Achieving a truly clean surface involves navigating several potential issues. An awareness of these pitfalls is crucial for obtaining reliable data.
The Risk of Over-Polishing
While polishing is essential for precision, excessive or aggressive polishing can alter the surface's morphology and roughness. This can change the effective surface area and affect experimental results, so always use a consistent, gentle technique.
The Fallacy of Visual Cleanliness
An electrode that looks clean to the naked eye may still have an invisible molecular film of contaminants. For sensitive measurements, you must trust the polishing protocol, not just a visual inspection.
Ignoring Regular Calibration
The electrode's response can change over time due to subtle surface changes. For any quantitative work, regular calibration is necessary to ensure the accuracy and reproducibility of your measurements.
How to Apply This to Your Goal
Your maintenance strategy should align with your experimental needs.
- If your primary focus is routine qualitative analysis: Regular visual inspection and basic cleaning with a solvent wipe before each use are often sufficient.
- If your primary focus is high-precision quantitative measurement: The full pre-treatment protocol, including methodical polishing and rinsing, is non-negotiable before every critical experiment.
- If you are storing the electrode long-term: Ensure it is completely clean and dry, then place it in a dedicated, airtight container to prevent atmospheric contamination.
Ultimately, a disciplined approach to electrode care is the foundation of trustworthy and reproducible electrochemical data.
Summary Table:
| Aspect | Key Action | Purpose |
|---|---|---|
| Prevention | Avoid oils, solvents, sulfur/chlorine; handle with care. | Shield the soft gold surface from chemical and physical damage. |
| Basic Cleaning | Wipe with soft cloth and pure water/ethanol/acetone. | Remove minor surface dirt and organic films. |
| Precision Cleaning | Polish with alumina powder in a figure-8 motion. | Create a uniform, contaminant-free surface for quantitative work. |
| Post-Cleaning | Rinse with ethanol/deionized water; dry thoroughly. | Eliminate polishing residue and prevent solvent interference. |
Achieve Peak Electrode Performance with KINTEK
Your electrochemical experiments demand reliable and pristine electrodes. KINTEK specializes in high-quality lab equipment and consumables, providing the tools and expertise to support your precise research needs. From durable electrode materials to specialized cleaning supplies, we help you maintain the integrity of your instruments for reproducible, high-quality data.
Let us help you ensure your lab's success. Contact our experts today to discuss your specific requirements and discover the right solutions for your laboratory.
Visual Guide
Related Products
- Gold Electrochemical Sheet Electrode Gold Electrode
- Gold Disc Electrode
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Metal Disc Electrode Electrochemical Electrode
People Also Ask
- What are the disadvantages of gold electrodes? Key Limitations for Your Lab Projects
- What is the operating principle of a gold disc electrode in an electrochemical system? Unlock Precision with a Stable Interface
- What are the key aspects of maintaining and caring for a gold plate electrode? Preserve Performance and Extend Lifespan
- What are gold electrodes used for? Achieve Unmatched Sensitivity in Biosensing and Research
- In what scenarios should a gold plate electrode be dedicated to a single user or purpose? Ensure Patient Safety and Data Integrity



















