The fundamental precaution for a gold plate electrode is to treat it with meticulous care, recognizing its inherent physical fragility. Because gold is an exceptionally soft metal and these electrodes are often fabricated as thin sheets, they can be easily bent, scratched, or deformed by even minor physical force, which compromises their performance.
The core principle is simple: the electrode's value lies in its pristine surface. Any mechanical damage, from a microscopic scratch to a visible bend, alters its electroactive surface area and geometry, directly undermining the accuracy and reproducibility of your measurements.

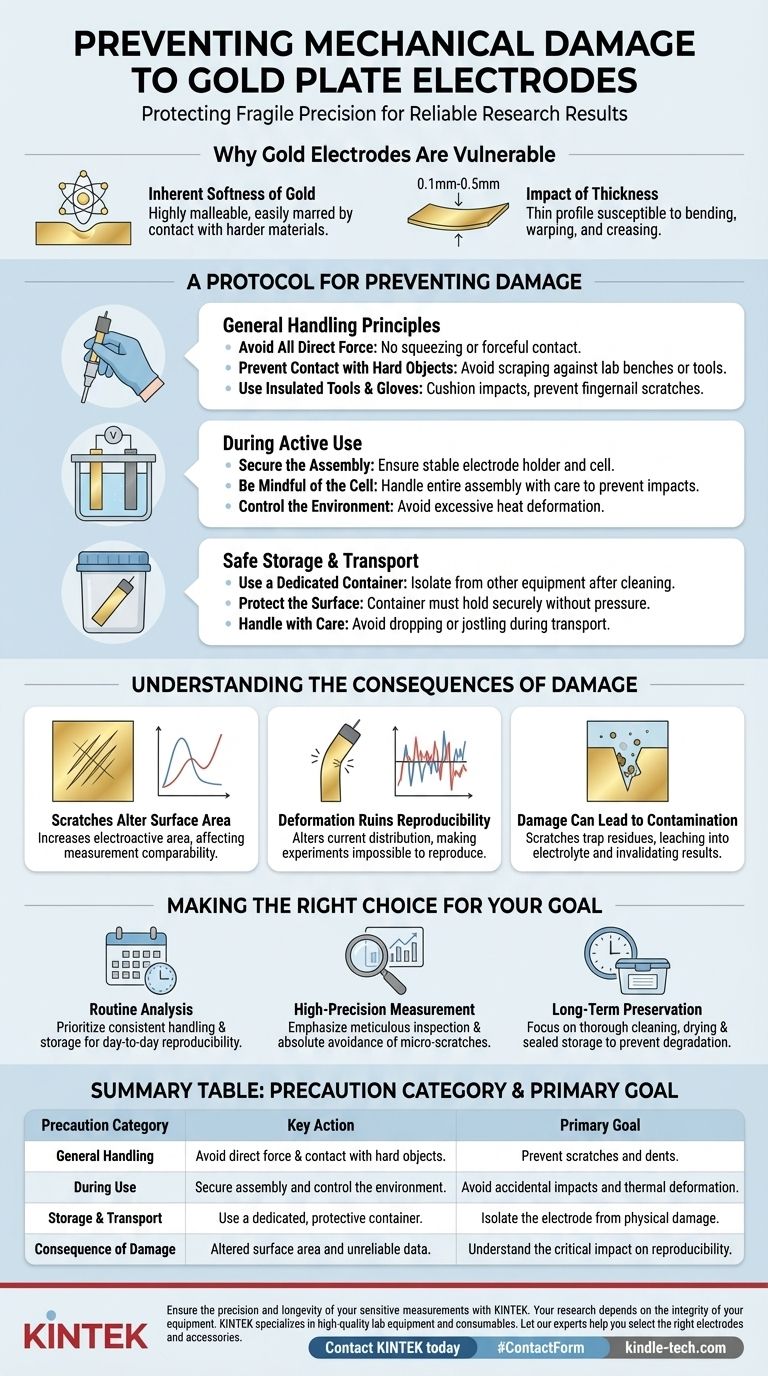
Why Gold Electrodes Are So Vulnerable
Understanding the material's properties is the first step toward proper handling. The need for extreme care is not arbitrary; it is rooted in the physical nature of gold and the typical construction of these electrodes.
The Inherent Softness of Gold
Gold is one of the most malleable metals. This softness means its surface can be easily marred. Contact with harder materials, including metal tools, abrasive surfaces, or even the edge of a glass beaker, can create scratches or dents.
The Impact of Thickness
Gold plate electrodes are typically very thin sheets, often ranging from 0.1mm to 0.5mm thick. This thin profile makes them highly susceptible to bending, warping, or creasing from impacts, squeezing, or improper handling.
A Protocol for Preventing Damage
A systematic approach during every stage—handling, operation, and storage—is essential for preserving the electrode's integrity.
General Handling Principles
- Avoid All Direct Force: Never apply squeezing pressure or forceful contact to the electrode plate. Handle it gently, ideally by its connecting components rather than the plate itself.
- Prevent Contact with Hard Objects: Ensure the electrode surface does not scrape against or strike any hard objects. This includes lab benches, equipment stands, or other tools.
- Use Insulated Tools and Gloves: While primarily an electrical safety measure, using soft, insulated tools and gloves can also help cushion the electrode from accidental impacts and prevent scratches from fingernails or jewelry.
During Active Use
- Secure the Assembly: Ensure the electrode holder and electrolytic cell are stable. A wobbly stand can lead to accidental collisions that damage the fragile electrode plate.
- Be Mindful of the Cell: The electrolytic cell, especially if made of glass, is also fragile. Handle the entire assembly with care to prevent impacts that could damage both the cell and the electrode within it.
- Control the Environment: While not strictly mechanical, high temperatures can cause the thin gold plate to deform. Avoid using or storing the electrode in excessively hot environments.
Safe Storage and Transport
- Use a Dedicated Container: After cleaning and drying, always store the electrode in a dedicated, purpose-built container. This isolates it from other equipment and prevents accidental contact.
- Protect the Surface: The container should be designed to hold the electrode securely without putting pressure on the plate surface.
- Handle with Care: During transport, even within its protective case, avoid dropping or jostling the electrode.
Understanding the Consequences of Damage
Failing to prevent mechanical damage has direct and significant consequences for your work. This is not merely a cosmetic issue; it fundamentally impacts the electrode's function.
Scratches Alter Surface Area
Microscopic scratches and gouges increase the electrode's electroactive surface area. This change means your results will no longer be comparable to previous measurements or theoretical calculations based on a smooth, defined geometric area.
Deformation Ruins Reproducibility
A bent or warped electrode plate alters the current and potential distribution across its surface. This can introduce artifacts into your data and makes it nearly impossible to reproduce experiments reliably, which is the cornerstone of sound scientific work.
Damage Can Lead to Contamination
Scratches and crevices can trap residues from previous experiments or cleaning agents. This trapped material can then leach out during subsequent use, contaminating your electrolyte and invalidating your results.
Making the Right Choice for Your Goal
Adopting a careful protocol is non-negotiable. Your specific focus will determine which precautions are most critical to emphasize.
- If your primary focus is routine analysis: Prioritize consistent handling and storage procedures to ensure day-to-day reproducibility of your results.
- If your primary focus is high-precision measurement: Emphasize meticulous surface inspection before each use and absolute avoidance of any contact that could create micro-scratches.
- If your primary focus is long-term preservation: Focus on thorough post-use cleaning, complete drying, and storage in a sealed, protective container to prevent both mechanical and chemical degradation over time.
Ultimately, treating your gold electrode with deliberate care is the foundation for generating reliable and accurate data.
Summary Table:
| Precaution Category | Key Action | Primary Goal |
|---|---|---|
| General Handling | Avoid direct force & contact with hard objects. | Prevent scratches and dents. |
| During Use | Secure assembly and control the environment. | Avoid accidental impacts and thermal deformation. |
| Storage & Transport | Use a dedicated, protective container. | Isolate the electrode from physical damage. |
| Consequence of Damage | Altered surface area and unreliable data. | Understand the critical impact on reproducibility. |
Ensure the precision and longevity of your sensitive measurements with KINTEK.
Your research depends on the integrity of your equipment. A damaged gold electrode can compromise your data's accuracy and reproducibility. KINTEK specializes in high-quality lab equipment and consumables, serving the precise needs of laboratories like yours.
Let our experts help you select the right electrodes and accessories, and provide guidance on best practices for handling and maintenance.
Contact KINTEK today to discuss your laboratory requirements and discover how we can support your pursuit of reliable, high-quality results.
Visual Guide
Related Products
- Gold Electrochemical Sheet Electrode Gold Electrode
- Gold Disc Electrode
- Metal Disc Electrode Electrochemical Electrode
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Graphite Disc Rod and Sheet Electrode Electrochemical Graphite Electrode
People Also Ask
- What are the key aspects of maintaining and caring for a gold plate electrode? Preserve Performance and Extend Lifespan
- What are the disadvantages of gold electrodes? Key Limitations for Your Lab Projects
- What are the performance characteristics of a gold plate electrode? Unmatched Stability for Reliable Data
- In what scenarios should a gold plate electrode be dedicated to a single user or purpose? Ensure Patient Safety and Data Integrity
- How should contamination of a gold plate electrode be prevented and managed? Essential Care for Reliable Data



















